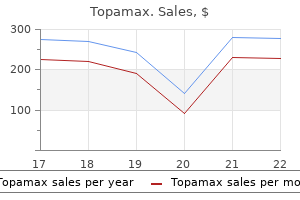
Topamax 100mg without a prescription
This reversal of the ventricular-aortic pressure gradient in the presence of continuous flow of blood from the left ventricle to the aorta is the result of storage of potential energy in the stretched arterial walls medications rights buy topamax now. This stored potential energy causes blood flow from the left ventricle into the aorta to decelerate. The peak of the flow curve coincides with the point at which the left ventricular pressure curve intersects the aortic pressure curve during ejection. Thereafter, flow decelerates (continues to decrease) because the pressure gradient has been reversed. The c wave is caused by impact of the common carotid artery with the adjacent jugular vein and, to some extent, by the abrupt closure of the tricuspid valve in early ventricular systole. Except for the c wave, the Closure of the aortic valve produces the characteristic incisura (notch) on the descending limb of the aortic pressure curve, and it also produces the second heart sound (with some vibrations evident on the atrial pressure curve). It is characterized by a precipitous fall in ventricular pressure without a change in ventricular volume. At this point, the blood that returned to the atria during the previous ventricular systole is abruptly released into the relaxing ventricles. The rapid flow of blood from atria to relaxing ventricles produces transient decreases in atrial and ventricular pressures and a sharp increase in ventricular volume. Diastasis Rapid Filling Phase the rapid ventricular filling phase is followed by a phase of slow ventricular filling called diastasis. During diastasis, blood returning from the peripheral veins flows into the right ventricle and blood from the lungs flows into the left ventricle. The transfer of blood from atrium to ventricle achieved by atrial contraction completes the period of ventricular filling. Throughout ventricular diastole, atrial pressure barely exceeds ventricular pressure. Because there are no valves at the junction of the venae cavae and right atrium or at the junction of the pulmonary veins and left atrium, atrial contraction may force blood in both directions. However, little blood is actually pumped back into the venous tributaries during the brief atrial contraction, mainly because of the inertia of the inflowing blood. At slow heart rates, filling practically ceases toward the end of diastasis, and atrial contraction contributes little additional filling. During tachycardia, however, diastasis is abbreviated and the atrial contribution can become substantial. Should tachycardia become so severe that the rapid filling phase is attenuated, atrial contraction assumes great importance in rapidly propelling blood into the ventricle during this brief period of the cardiac cycle. If the period of ventricular relaxation is so brief that filling is seriously impaired, even atrial contraction cannot provide adequate ventricular filling. The initial decrease in left ventricular pressure (from points A to B), despite the rapid inflow of blood from the left atrium, is attributed to progressive ventricular relaxation and distensibility. During the remainder of diastole (from points B to C), the increase in ventricular pressure reflects ventricular filling and changes in the passive elastic characteristics of the ventricle. Note that only a small increase in pressure accompanies the substantial increase in ventricular volume during diastole (from points B to C). The small pressure increase reflects the compliance of the left ventricle during diastole. The small increase in pressure just before the mitral valve closes (to the left of point C) is caused by the contribution of atrial contraction to ventricular filling. With isovolumic contraction (from points C to D), pressure rises steeply, but ventricular volume does not change because the mitral and aortic valves are both closed. When the aortic valve opens (point D), and during the first (rapid) phase of ejection (from points D to E), the large reduction in volume is associated with a steady increase in ventricular pressure. This reduction in volume is followed by reduced ejection (from points E to F) and a small decrease in ventricular pressure. Closure of the aortic valve (point F) is followed by isovolumic relaxation (from points F to A), which is characterized by a sharp drop in pressure. Ventricular volume does not change during the interval between closing of the aortic valve and opening of the mitral valve (from points F to A) because both the mitral and aortic valves are closed. Several key cardiovascular system parameters are evident on a left ventricular pressure-volume loop (P-V loop), or may be calculated from it. In this subject, partial occlusion of the inferior vena cava reduced the preload of the left ventricle on successive beats (as the inflow of blood to the left ventricle was reduced, as a consequence of the reduced return of blood to the right ventricle) and the effect was successively smaller stroke volume of the left ventricle. This illustrates, in humans, the operation of the FrankStarling law of the heart (Chapter 13), whereby changes in preload (left ventricular volume) change myocardial fiber length, and thus the strength of the subsequent contraction, as well as stroke volume produced. If contractility of the heart is increased, as in the action of norepinephrine, the slope of the end-systolic P-V relation becomes steeper, and the entire cardiac function curve shifts upward (solid red line), which reflects the fact that the ventricle is now able to produce a larger stroke volume at a given preload. The increased stroke volume at any given preload is largely produced by a decreased end-systolic volume: Hearts with increased contractility are able to "squeeze down" to a greater extent. Conversely, if the ventricle is damaged, as after cardiac ischemia, or if its contractility is otherwise reduced from normal (as after calcium channel blockade), the cardiac function curve is shifted downward (green line), which reflects a reduced stroke volume at any given preload. The cardiac function curve is also known as ventricular function curves or Starling curves. To assess the integrated functioning of the cardiovascular system (Chapter 19), cardiac output, rather than stroke volume, is usually measured; preload of the heart is considered to be the filling pressure of the right ventricle, typically measured as mean right atrial pressure (Pra), or central venous pressure. Measurement of Cardiac Output the Fick Principle In 1870 the German physiologist Adolph Fick contrived the first method for measuring cardiac output in intact animals and people. The basis for this method, called the Fick principle, is simply an application of the law of conservation of mass. To determine cardiac output by this method, three values must be known: (1) O2 consumption of the body, (2) the O2 concentration in pulmonary venous blood ([O2]pv), and (3) the O2 concentration in pulmonary arterial blood ([O2]pa). O2 consumption is computed from measurements of the volume and O2 content of expired air over a given interval. Because the O2 concentration of peripheral arterial blood is essentially identical to that in the pulmonary veins, [O2]pv is determined with a sample of peripheral arterial blood withdrawn by needle puncture. The compositions of pulmonary arterial blood and mixed systemic venous blood are virtually identical to one another. Samples for O2 analysis are obtained from the pulmonary artery or right ventricle through a catheter. A very flexible catheter with a small balloon near the tip can be inserted into a peripheral vein. By monitoring the pressure changes, the physician can advance the catheter tip into the pulmonary artery. The Fick principle is also used to estimate the O2 consumption of organs when blood flow and the O2 content of arterial and venous blood can be determined. Algebraic rearrangement reveals that O2 consumption equals blood flow multiplied by the difference in the arteriovenous O2 concentration. For example, if blood flow through one kidney is 700 mL/minute, the arterial O2 content is 0. In the clinic, cardiac output is most commonly measured noninvasively with Doppler echocardiography. Obtaining the cross-sectional area of the aorta (also measured by echocardiography) allows the volume of blood ejected in a single beat. Multiplying stroke volume by the heart rate then yields a value for cardiac output in liters per minute. The rate of O2 delivery to the lungs (q1) equals the O2 concentration in pulmonary arterial blood ([O2]pa), multiplied by pulmonary arterial blood flow (Q), which equals cardiac output; that is, Equation 16. The rate at which O2 is carried away by the pulmonary veins (q3) equals the O2 concentration in pulmonary venous blood ([O2]pv), multiplied by total pulmonary venous flow, which is virtually equal to pulmonary arterial blood flow (Q); that is, Equation 16. The O2 content of cardiac venous blood is normally low (5 mL/ dL), and the myocardium can receive little additional O2 by further extraction of O2 from coronary blood. Therefore, increased O2 demands of the heart must be met mainly by an increase in coronary blood flow (see Chapter 17). In experiments in which the heartbeat is arrested but coronary perfusion is maintained, O2 consumption falls to 2 mL/ minute/100 g or less, which is still six to seven times greater than the O2 consumption of resting skeletal muscle. Left ventricular work per beat (stroke work) is approximately equal to the product of stroke volume and the mean aortic pressure against which the blood is ejected by the left ventricle. Thus myocardial O2 consumption may not be well correlated with overall cardiac work. The magnitude and duration of left ventricular pressure are correlated with left ventricular O2 consumption. The work of the right ventricle is one-seventh that of the left ventricle because pulmonary vascular resistance is much less than systemic vascular resistance. Cardiac Efficiency the efficiency of the heart may be calculated as the ratio of the work accomplished to the total energy used.
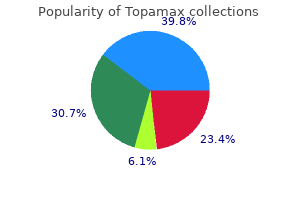
Discount 200 mg topamax with amex
Overall treatment 12mm kidney stone purchase generic topamax line, gas velocity is blunted because energy is consumed in the process of generating eddies and chaotic movement. As a consequence, higher driving pressure is needed to support a given turbulent flow than to support a similar laminar flow. Whether flow through a tube is laminar or turbulent depends on the Reynolds number. The Reynolds number (Re) is a dimensionless value that expresses the ratio of two dimensionally equivalent terms (kinematic/viscosity), as seen in the equation: Equation 22. In straight tubes, turbulence occurs when the Reynolds number is greater than 2000. From this relationship it can be seen that turbulence is most likely to occur when the average velocity of the gas flow is high and the radius is large. In contrast, a low-density gas such as helium is less likely to cause turbulent flow. This is clinically relevant in states of increased airway resistance where a decrease in gas density. Although these relationships apply well to smooth cylindrical tubes, application of these principles to a complicated system of tubes such as the airways is difficult. As a result, much of the flow in the airways demonstrates characteristics of both laminar and turbulent flow. In the trachea, for example, even during quiet breathing the Reynolds number is greater than 2000. Turbulence is also promoted by the glottis and vocal cords, which produce some irregularity and obstruction in the airways. As gas flows distally the total cross-sectional area increases dramatically, and gas velocities decrease significantly. As a result, gas flow becomes more laminar in the smaller airways even during maximal ventilation. Overall the gas flow in the larger airways (nose, mouth, glottis, and bronchi) is turbulent, whereas the gas flow in the smaller airways is laminar. Laminar flow is silent, which is why it is difficult to "hear" small airway disease with a stethoscope. The reason for this is twofold: (1) airflow velocity decreases substantially as the effective cross-sectional area increases. The resistance of airways in parallel is the inverse of the sum of the individual resistances; therefore the overall contribution to resistance of the small airways is very small. If the tubes are in series, the total resistance (Rtot) is the sum of the individual resistances: R tot Equation 22. Thus as airway diameter decreases, the resistance offered by each individual airway increases, but the large increase in the number of parallel pathways and cross-sectional area reduces the resistance at each generation of branching. In moving from the trachea toward the alveolus, individual airways become smaller while the number of airway branches increases dramatically. In fact, however, the major site of resistance along the bronchial tree is in the first eight generations of airways. Because the small airways contribute so little to total lung resistance, measurement of airway resistance is a poor test for detecting small airway obstruction. In contrast, stimulation of sympathetic nerves and release of the postganglionic neurotransmitter norepinephrine inhibits airway constriction. Reflex stimulation of the vagus nerve by inhalation of smoke, dust, cold air, or other irritants can also result in airway constriction and coughing. These agents act directly on airway smooth muscle to cause constriction and an increase in airway resistance. Inhalation of methacholine, a derivative of acetylcholine, is used to diagnose airway hyperresponsiveness, which is one of the cardinal features of certain asthma phenotypes. Although everyone is capable of responding to methacholine, airway obstruction develops in individuals with asthma at much lower concentrations of inhaled methacholine. Conductance (L/sec/cm H2O) Measurement of Expiratory Flow Measurement of expiratory flow rates and expiratory volumes is an important clinical tool for evaluating and monitoring respiratory diseases. Results from individuals with suspected lung disease are compared with results predicted from normal healthy volunteers. Predicted or normal values vary with age, sex, ethnicity, height, and to a lesser extent, weight (Table 22. Abnormalities in values indicate abnormal pulmonary function and can be used to predict abnormalities in gas exchange. These values can detect the presence of abnormal lung function long before respiratory symptoms develop, and they can be used to determine disease severity and the response to therapy. Increasing lung volume increases the caliber of the airways because it creates a positive transairway pressure. As a result, resistance to airflow decreases with increasing lung volume and increases with decreasing lung volume. Other factors that increase airway resistance include airway mucus, edema, and contraction of bronchial smooth muscle, all of which decrease the caliber of the airways. When scuba diving, gas density rises and results in an increase in airway resistance; this increase can cause problems for individuals with asthma and obstructive pulmonary disease. Breathing a low-density gas such as an oxygen-helium mixture results in a decrease in airway resistance and has been exploited in the treatment of status asthmaticus, a condition associated with increased airway resistance due to a combination of bronchospasm, airway inflammation, and hypersecretion of mucus. A ratio less than 70% suggests Neurohumoral Regulation of Airway Resistance In addition to the effects of disease, airway resistance is regulated by various neural and humoral agents. In thespirogramthat is reportedin clinical settings, exhaledvolume increases from thebottomofthetracetothetop(A). A flow-volume curve or loop is created by displaying the instantaneous flow rate during a forced maneuver as a function of the volume of gas. Expiratory flow rates are displayed above the horizontal line, and inspiratory flow rates are displayed below the horizontal line. Determinants of Maximal Flow the shape of the flow-volume loop reveals important information about normal lung physiology that can be altered by disease. Inspection of the flow-volume loop reveals that the maximum inspiratory flow is the same or slightly greater than the maximum expiratory flow. This opposes the force generated by the inspiratory muscles and reduces maximum inspiratory flow. However, airway resistance decreases with increasing lung volume as the airway caliber increases. This is known as expiratory flow limitation and can be demonstrated by asking an individual to perform three forced expiratory maneuvers with increasing effort. However, the flow rates at lower lung volumes converge; this indicates that with modest effort, maximal expiratory flow is achieved. For this reason, expiratory flow rates at lower lung volumes are said to be effort independent and flow limited because maximal flow is achieved with modest effort, and no amount of additional effort can increase the flow rate beyond this limit. In contrast, events early in the expiratory maneuver are said to be effort dependent; that is, increasing effort generates increasing flow rates. In general the first 20% of the flow in the expiratory flow-volume loop is effort dependent. Flow Limitation and the Equal Pressure Point Why is expiratory flow limited and reasonably effort independent Factors that limit expiratory flow are important because many lung diseases affect these factors and thus affect the volume and speed with which air is moved into and out of the lung. Flow limitation occurs when the airways, which are intrinsically floppy distensible tubes, become compressed. The airways become compressed when the pressure outside the airway exceeds the pressure inside the airway. The airways are shown as tapered tubes because the total or collective airway crosssectional area decreases from the alveoli to the trachea. Because there is no flow, the pressure inside the airways is zero and the pressure across the airways (Pta, transairway pressure) is +30 cm H2O [Pta = Pairway - Ppl = 0 - (-30 cm H2O)]. This positive transpulmonary and transairway pressure holds the alveoli and airways open. When an active exhalation begins and the expiratory muscles contract, pleural pressure rises to +60 cm H2O (in this example).
Purchase 100 mg topamax visa
Inappropriate technique or poor tissue handling may limit the diagnostic yield of a skin biopsy; accordingly medications ending in zole buy cheapest topamax, clinicians must have an appreciation of the principles of histologic examination. However, this union exists not only because of overlapping subject matter, but because dermatology Site selection Often, the first step in performing a biopsy is to identify an unadulterated primary lesion. Lesions with obfuscating secondary features, such as those resulting from rubbing or traumatic injury. Strongyloidiasis ** ** * not a single site as in cellulitis, necrotizing fasciitis ** more likely in immunocompromised patient *** early on, more serious drug reactions. Immature lesions may not yet manifest characteristic histopathologic changes, and older lesions may be compromised by secondary features. Of course, there are exceptions to this general principle, such as the sampling of early lesions of cutaneous small vessel vasculitis (<24 hours old) or immunobullous diseases, especially when performing direct immunofluorescence. For example, in atrophoderma an incisional biopsy should include both affected and unaffected skin and be sectioned longitudinally, so that subtle differences can be detected (see Ch. In ulcers, nonspecific inflammation of vessels underneath the wound may be misinterpreted as a primary vasculitis, but in a biopsy specimen that includes the surrounding skin, the "vasculitis" disappears a few millimeters away from the ulcer. Ultimately, selection of a proper biopsy site will always be influenced by knowledge of the suspected underlying pathology. Evidence suggests that when properly performed, the diagnostic value of a deep shave may rival that of an incisional/excisional procedure25. In this regard, curettage is less desirable for diagnostic purposes, and it is not appropriate for pigmented lesions that are suspicious for melanoma or for neoplasms of uncertain etiology. For optimal results, the technique employed must encapture tissue from the level of the skin or subcutaneous tissue where the pathologic changes are anticipated, while simultaneously balancing concerns of cosmesis and morbidity. For example, if panniculitis is suspected, a shave would not provide the appropriate tissue to establish or refute such a diagnosis (Table 0. If the sampled lesion can be contained in the punch, then the concern regarding sampling error is rendered moot. It is controversial as to whether punch biopsies, even if performed in a "stacked" fashion, can provide adequate tissue for assessment of deeply infiltrating tumors or panniculitis. Studies suggest that partial punch samplings of melanocytic lesions can lead to misdiagnosis or to erroneous staging and therefore should not be performed26. Handling of the specimen after biopsy Skin specimens must be handled carefully upon extirpation. For example, excessive lateral pressure by forceps on small punch biopsy specimens can distort cellular infiltrates, particularly lymphomas and Merkel cell carcinoma, creating so-called "crush" artifact. These two cell types are also subject to dessication artifact when the biopsy specimen is placed onto gauze rather than into formalin solution. Fixation in paraformaldehyde and glutaraldehyde in a cacodylate buffer is required for electron microscopy. To obtain the most accurate histopathologic assessment, all biopsy specimens sent to a dermatopathologist should be accompanied by relevant clinical data such as: age and sex of the patient, anatomic site(s) involved, pertinent physical findings, and a suspected clinical differential diagnosis. Prior treatments that might impact upon the histologic findings should be disclosed. Inclusion of drawings or clinical photographs may prove useful, especially in difficult or complex cases. It is important to remember that even though an entity has lichenoid features under the microscope. Also, some degree of lichenoid inflammation may be associated with a variety of benign and malignant neoplasms, such as lichenoid keratoses and melanoma, respectively. In these instances, the lichenoid inflammation represents an immunological response to the tumor. The algorithmic approach of pattern analysis is reproducible, and it minimizes subjectivity. However, the method has two important limitations, namely, it is based on artificial disease categories and it cannot include every possible pattern. Furthermore, while pattern analysis clearly narrows the differential diagnosis, a final assessment may require clinical correlation and/or ancillary laboratory testing, imaging, or genetic testing29. Also, the histopathologic appearance of skin disease may vary based upon the temporal course. The histologic findings may be altered by previous treatment(s) or by secondary changes such as rubbing, scratching, or infection. Lastly, pattern analysis is not only applicable to inflammatory skin diseases, but is also used for neoplastic processes. Spongiosis (intercellular edema) is a nonspecific morphologic alteration that is observed in a variety of skin conditions. The degree of spongiosis may vary from microscopic foci to grossly visible vesicles or intraepidermal bullae. There is often associated exocytosis of inflammatory cells, with migration from the vasculature into the epidermis. Spongiotic dermatoses may be further subdivided into acute, subacute and chronic forms. Parakeratosis, a histologic equivalent of scale, often overlies subacute spongiotic dermatitis. In chronic spongiotic dermatitis, the spongiosis may be more difficult to appreciate, being instead overshadowed by epidermal acanthosis (thickening of the epidermis). Also, a predominance of certain inflammatory cells in association with spongiosis, such as eosinophils or neutrophils, may serve as a clue to a hypersensitivity component or infectious process, respectively. Lastly, it is important to recognize that multiple cutaneous disorders with eczematous features, such as allergic contact dermatitis, atopic dermatitis, nummular dermatitis and seborrheic dermatitis, may have histologic evidence of spongiosis, but this pattern is not exclusive to those diseases. Ten patterns defined Over the past several decades, different classification schema based upon pattern analysis have emerged. The number of patterns in any schema has varied from 9 to 28 or more, but in this introductory chapter, 10 major patterns will be discussed. Psoriasiform dermatoses can be further subdivided into those diseases that are exclusively psoriasiform and those that are associated with another pattern. Pseudoepitheliomatous hyperplasia represents a related, but irregular, hyperplasia of the epidermis and/or adnexal structures. It may Psoriasiform dermatitis Basic Principles of Dermatology 15 lesion (excisional) via a scalpel, using standard surgical techniques (see Ch. Optimal biopsy techniques based upon the suspected cutaneous disease are outlined in Table 0. Traditionally, perivascular dermatitis has been subdivided into "superficial" and "superficial and deep" variants, and while this division has some diagnostic value, considerable overlap exists. In addition, inflammatory skin diseases can exhibit a spectrum of findings, depending in part upon severity, as well as the duration of an individual lesion (acute vs chronic). There are disorders without detectable changes within the epidermis, such as deep gyrate erythemas (see Ch. As with spongiotic dermatitis, psoriasiform dermatitis is a histologic concept, not a specific clinical diagnosis, and its presence mandates consideration of a variety of skin diseases that share this particular constellation of histopathologic findings. Vesiculobullous and pustular dermatoses the concept of intraepidermal vesiculation due to spongiosis has been addressed above, but other disease mechanisms may lead to formation of intraepidermal vesicles or bullae. For example, superficial (subcorneal) acantholysis may favor pemphigus foliaceus, while acantholysis within the deeper portion of the epidermis is more characteristic of pemphigus vulgaris. Ballooning degeneration refers to intracellular edema in response to cytotoxic events. When ballooning is severe, keratinocytes rupture, resulting in reticular degeneration and epidermal necrosis. Pustule formation (the intraepidermal accumulation of neutrophils) may be seen in a variety of infectious and non-infectious skin diseases. In a resolving pustule, the neutrophils or their remnants may even appear within a scale-crust in the cornified layer. In both vesiculobullous and pustular dermatoses, autoimmune and non-autoimmune mechanisms.
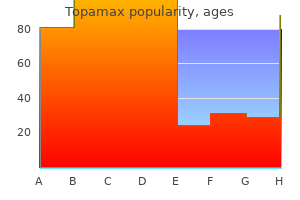
Cheap 200 mg topamax otc
In addition treatment using drugs buy topamax 200 mg mastercard, a small amount of water traverses the capillary wall via pinocytosis by endothelial cells. The driving forces for fluid (water) movement across the capillary wall are hydrostatic pressure and oncotic pressure. Capillary fluid movement is discussed in detail in Chapter 17; in brief, hydrostatic pressure within the capillary (as a result of the pumping of the heart and the effect of gravity on the column of blood in the vessels feeding a capillary) is a force that causes fluid to move out of the capillary. Hydrostatic pressure in the surrounding interstitial tissue opposes the effect of the capillary hydrostatic pressure. The oncotic pressure of the plasma in the capillary tends to draw fluid from the interstitium into the capillary. Thus the amount of fluid moving across the wall of the capillary is determined as follows: Equation 2. Depending on the magnitude of these forces, fluid may move out of the capillary or into the capillary. The compositions of the various body fluid compartments differ; however, as described later, the osmolalities of the fluid within these compartments are essentially identical. Because of this rapid redistribution of water, measuring the osmolality of plasma or serum,c which is easy to do, reveals the osmolality of the other body fluid compartments. For example, if a blood sample is obtained from an individual, and the [Na+] of the serum is 145 mEq/L, its osmolality can be estimated as follows: Equation 2. Consequently, in trying to understand the physiology of fluid shifts between body fluid compartments, it can be assumed that while water moves freely between the compartments, there is little net movement of solutes. With regard to osmolality and the concentrations of other molecules and ions, the osmolality and concentrations in plasma and serum are virtually identical. When electrolyte solutions are infused by this route, equilibration between plasma and interstitial fluid is rapid. Accordingly,serumosmolalitycanbeestimated asfollows: Serum osmolality = 2(serum [Na +]) + [glucose] [urea] + 18 2. Therefore, there is no net movement of the infused isotonic NaCl solution into cells. The major difference between the interstitial fluid and plasma is that the latter contains significantly more protein. This section is a review of the mechanisms by which cells maintain their intracellular ionic environment and their membrane potential and by which they control their volume. Ionic Composition of Cells the intracellular ionic composition of cells varies from tissue to tissue. For example, the intracellular composition of neurons is different from that of muscle cells, both of which differ from that of blood cells. For example, as described in Chapter 1, a number of solute carriers couple the transport of Na+ to that of other ions or molecules. For example, the activity of some enzymes is pH dependent; therefore, intracellular pH must be regulated. In addition, the intracellular composition of other electrolytes is similarly held within a narrow range. This is necessary for the establishment of the membrane potential, a cell property especially important for the normal function of excitable cells. Finally, the volume of cells must be maintained because shrinking or swelling of cells can lead to cell damage or death. Similarly, the inwardly directed Na+ gradient drives the secondary active extrusion of H+ from the cell and thus contributes to the maintenance of intracellular pH. As might be expected, the contribution of various electrogenic transporters to the Vm is highly variable from cell to cell. Similarly, the contribution of other electrogenic transporters, such as the 3Na+-Ca++ antiporter and the Na+-glucose symporter is minimal. As described in Chapter 5, rapid changes in ion channel activity underlies the action potential in neurons and other excitable cells, such as those of skeletal and cardiac muscle (see Chapters 12 and 13). As described in Chapter 1, this current can be measured, even at the level of a single channel. By convention, the current generated by the movement of cations into the cell, or the movement of anions out of the cell, is defined as negative current. Conversely, the movement of cations out of the cell, or the movement of anions into the cell, is defined as positive current. Also by convention, the magnitude of the Vm is expressed in relation to the outside of the cell; thus for a cell with a Vm of -80 mV, the interior of the cell is electrically negative in relation to the outside of the cell. The current carried by ions moving through a channel depends on the driving force for that ion and on the conductance of the channel. As described in Chapter 1, the driving force is determined by the energy in the concentration gradient for the ion across the membrane (Ei), as calculated by the Nernst equation (Eq. For a cell, the conductance of the membrane to a particular ion (Gi) is determined by the number of ion channels in the membrane and by the amount of time each channel is in the open state. To understand what determines the magnitude of the Vm, it is important to recognize that any transporter that transfers charge across the membrane has the potential to influence the Vm. As the K+ conductance returns to its baseline value, Vm returns to its resting value of -70mV. For most cells at rest, the membrane has a high conductance to K+, and thus the Vm approximates E K +. For example, if the intracellular [K+] is 120 mEq/L and the extracellular [K+] is 4 mEq/L, E K + has a value of -90. Conversely, if the extracellular [K+] is decreased to 2 mEq/L, E K + becomes -109. When an action potential is initiated, Na+-channels open and the membrane is now conductive predominantly to Na+. Regulation of Cell Volume As already noted, changes in cell volume can lead to cell damage and death. Most cells are highly permeable by water because of the presence of aquaporins in their plasma membranes. As discussed in Chapter 1, osmotic pressure gradients across the cell membrane that are generated by effective osmoles cause water to move either into or out of the cell, which result in changes in cell volume. Thus cells swell when placed in hypotonic solutions and shrink when placed in hypertonic solutions (see the section "Nonisotonic Cell Volume Regulation"). The Gibbs-Donnan effect occurs when a membrane separating two solutions can be permeated by some but not all of the molecules in solution. As noted previously, this effect accounts for the small differences in the ionic compositions of the plasma and the interstitial fluid. In this case, the capillary endothelium represents the membrane, and the plasma proteins are the molecules whose ability to permeate across the capillary is restricted. For cells, the membrane is the plasma membrane, and the impermeant molecules are the intracellular proteins and organic molecules. This increases the number of osmotically active particles in the compartment containing the impermeant anions, which in turn increases the osmotic pressure, and water thereby enters that compartment. For cells, the Gibbs-Donnan effect would increase the number of osmotically active particles in the cell, and result in cell swelling. Conversely with cell shrinking a regulatory volume increase response transports osmolytes into the cell, raising the intracellular osmotic pressure and thereby restoring cell volume to normal. These osmolytes include ions and organic molecules such as polyols (sorbitol and myo-inositol), methylamines (glycerophosphorylcholine and betaine), and some amino acids (taurine, glutamate, and -alanine). Cell swelling or shrinkage can result in cell damage or death, but many cells have mechanisms that limit the degree to which the cell volume changes. These mechanisms are particularly important for neurons, in which swelling within the confined space of the skull can lead to serious neurological damage. With cell swelling, a regulatory volume decrease response transports osmotically active particles (osmolytes) out of the cell, reducing the regulatory volume increase response results in the rapid uptake of NaCl and a number of organic osmolytes. These transporters use the energy in the Na+ and Cl- gradients to drive the secondary active uptake of these organic osmolytes into cells.
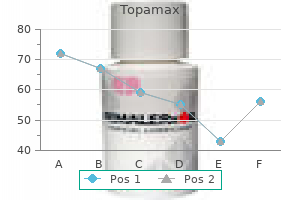
Discount topamax 200mg online
You can see the effect of this suppression by moving your finger back and forth in front of this text while tracking it with your eyes 340b medications buy cheap topamax 200 mg. Your finger will be in focus, but the words on this page will be part of the background scene and will become illegible as they slip along your retina. However, with a prolonged stimulus, the eyes will reach their mechanical limit, no further compensation will be possible, and the image will begin to slip on the retina. To avoid this situation, a fast saccade-like movement of the eyes occurs in the opposite direction, essentially resetting the eyes to begin viewing the visual scene again. Thus nystagmus can be defined as oscillatory or rhythmic movements of the eye in which there is a fast phase and a slow phase. The nystagmus is named according to the direction of the fast phase because the fast phase is more easily observed. Very rapid discrete movements that bring a particular region of the visual world onto the fovea are called saccades. For example, to read this sentence, you are making a series of saccades to bring successive words onto your fovea to be read. However, even in animals that lack a fovea, the eyes make saccades, and thus saccades may also be used to rapidly scan the visual environment. This spontaneous activity allows the antagonist muscle pairs to act in a push-pull manner, which increases the responsiveness of the system. That is, as motor neurons innervating one muscle are activated and cause increased contraction, those innervating its antagonist are inhibited, which leads to relaxation. These neurons project, via the medial longitudinal fasciculus, to medial rectus motor neurons in the contralateral oculomotor nucleus. Eye position Circuits Underlying the Vestibuloocular Reflex vestibular circuits, either in the periphery. There are separate circuits for rotational and translational movement of the head. The sensors for the former are the semicircular canals, and the sensors for the latter are the otoliths (the utricle and saccule). Note that only the major central circuits originating in the left horizontal canal and vestibular nuclei are shown; however, mirror image pathways arise from the right canal and vestibular nuclei. Control of the medial rectus muscle is achieved by abducens internuclear neurons that project from the abducens to the part of the oculomotor nucleus controlling the medial rectus muscle. Note the double decussation of this pathway results in aligning of the responses of functional synergists. Such coordination allows a target to be maintained on both foveae during eye movement and is necessary to maintain binocular vision without diplopia (double vision). However, when objects are close (<30 m), maintaining a target on both foveae requires non-identical movements of the two eyes. Such disjunctive, or vergence, movements are also necessary for fixation of both eyes on objects that are approaching or receding. It should be noted that when tracking an approaching object in addition to convergence movements, the lens accommodates for near vision, and pupillary constriction occurs. Neural Circuitry and Activity Underlying Eye Movement Motor Neurons of the Extraocular Muscles Three cranial nerve nuclei supply the extraocular muscles: oculomotor, trochlear, and abducens nuclei. These three nuclei are sometimes referred to collectively as the oculomotor nuclei; however, the context (the specific nucleus or all three) should be clear. Motor neurons for the ipsilateral medial and inferior recti, ipsilateral inferior oblique, and contralateral superior rectus muscles reside in the oculomotor nucleus; those for the contralateral superior oblique muscle reside in the trochlear nucleus; and those for the ipsilateral lateral rectus muscle are located in the abducens nucleus. These motor neurons form some of the smallest motor units (1: 10 nerve-to-muscle ratio), which is consistent with the very fine control needed for precise eye movement. Note that only the major pathways originating in the left vestibular nuclei are shown. For clarity, only the beginnings of mirror image pathways from the right vestibular nuclei are shown (dotted lines). Increased axonal thickness indicates increased activity; thinner axons indicate decreased activity in comparison with levels at rest (A). The depolarized hair cells cause increased activity in the left vestibular afferent fibers and thereby excite neurons of the left medial vestibular nucleus. These include excitatory neurons that project to the contralateral abducens nucleus and synapse with both motor neurons and internuclear neurons. Excitation of the motor neurons leads to contraction of the right lateral rectus muscle and rotation of the right eye to the right, whereas excitation of the internuclear neurons of the right abducens nucleus leads to excitation of the medial rectus motor neurons in the left oculomotor nucleus, thus causing the left eye to rotate to the right as well. Along the pathway starting with the inhibitory vestibular neurons that project from the left medial vestibular nucleus to the ipsilateral abducens nucleus, the activity of these cells leads to inhibition of motor neurons to the left lateral rectus muscle and motor neurons to the right medial rectus muscle (the latter via internuclear neurons to the right oculomotor nucleus). Consequently, these muscles relax, thereby facilitating rotation of the eyes to the right. Thus the eye is being pulled by the increased tension of one set of muscles and "pushed" by the release of tension in the antagonist set of muscles. As an exercise, work out the resulting changes in activity through these circuits. Remember that leftward head rotation hyperpolarizes the hair cells of the right canal, thereby leading to a decrease in right vestibular afferent activity and disfacilitation of the right vestibular nuclear neurons. Now, consider the commissural fibers that connect the two medial vestibular nuclei are excitatory but end on local inhibitory interneurons of the contralateral vestibular nucleus and thus inhibit the projection neurons of that nucleus. This pathway reinforces the actions of the contralateral vestibular afferent fibers on their target vestibular nuclear neurons. In the aforementioned example, commissural cells in the left vestibular nucleus are activated and therefore cause active inhibition of the right medial vestibular nuclei projection neurons, which reinforces the disfacilitation caused by the decrease in right afferent activity. In fact, this commissural pathway is powerful enough to modulate the activity of the contralateral vestibular nuclei even after unilateral labyrinthectomy, which destroys the direct vestibular afferent input to these nuclei. Parts of the vermis and flocculonodular lobe receive primary vestibular afferent fibers or secondary vestibular afferent fibers (axons of the vestibular nuclear neurons), or both, and in turn project back to the vestibular nuclei directly and via a disynaptic pathway involving the fastigial nucleus. Key brainstem centers for this reflex lie in the tegmentum and pretectal region of the rostral midbrain. Directionselective, motion-sensitive retinal ganglion cells are a major afferent source carrying visual information to these nuclei. In addition, input comes from primary and higher order visual cortical areas in the occipital and temporal lobes. The efferent connections of these nuclei are numerous and complex and not fully understood. There are projections to various precerebellar nuclei, including the inferior olivary nucleus and basilar pontine nuclei. In sum, via several pathways operating in parallel, activity ultimately arrives at the various oculomotor nuclei whose motor neurons are activated, and proper counterrotation of the eyes results. These burst neurons are capable of extremely high bursts of spikes (up to 1000 Hz). Moreover, the gaze center has neurons showing tonic activity and burst-tonic activity. Normally, both inhibitory and excitatory burst neurons are inhibited by omnipause neurons located in the nucleus of the dorsal raphe. When a saccade is to be made, activity from the frontal eye fields or the superior colliculus, or both, leads to inhibition of the omnipause cells and excitation of the burst cells on the contralateral side. The initial bursts of these neurons allow strong contraction of the appropriate extraocular muscles, which overcomes the viscosity of the extraocular muscle and enables rapid movement to occur. Visual information about target velocity is processed in a series of cortical areas, including the visual cortex in the occipital lobe, several temporal lobe areas, and the frontal eye fields. In the past, the frontal eye fields were thought to be related only to control of saccades, but more recent evidence has shown that there are distinct regions within the frontal eye fields dedicated to either saccade production or smooth pursuit. Indeed, there may be two distinct cortical networks, each specialized for one of these types of eye movement. Cortical activity from multiple cortical areas is fed to the cerebellum via parts of the pontine nuclei and nucleus reticularis tegmenti pontis. Specific areas in the cerebellum-namely, parts of the posterior lobe vermis, the flocculus, and the paraflocculus-receive this input, and they in turn project to the vestibular nuclei. Activity in the superior colliculus is related to computation of the direction and amplitude of the saccade.
Strontium Chloride (Strontium). Topamax.
- What is Strontium?
- Are there any interactions with medications?
- What other names is Strontium known by?
- Dosing considerations for Strontium.
- How does Strontium work?
- Dental cavities, osteoarthritis, and other conditions. There is also no reliable information that strontium contained in dietary supplements is effective for any condition.
- Are there safety concerns?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=97023
Purchase generic topamax pills
Normal pH is maintained by buffers within body fluids and by the coordinated action of the lungs symptoms urinary tract infection order topamax 100 mg line, liver, and kidneys. These waste products include urea (from amino acids), uric acid (from nucleic acids), creatinine (from muscle creatine), end products of hemoglobin metabolism, and metabolites of hormones. The kidneys eliminate these substances from the body at a rate that matches their production. Finally, the kidneys are important endocrine organs that produce and secrete renin, calcitriol, and erythropoietin. Renin is not a hormone but an enzyme that activates the renin-angiotensin-aldosterone system, which helps regulate blood pressure and Na+ and K+ balance. Calcitriol, a metabolite of vitamin D3, is necessary for normal absorption of Ca++ by the gastrointestinal tract and for its deposition in bone (see Chapter 36). As a result, Ca++ absorption by the intestine is decreased, which over time contributes to the bone formation abnormalities seen in patients with chronic renal disease. Another consequence of many kidney diseases is a reduction in erythropoietin production and secretion. In some instances the impairment in renal function is transient, but in many cases renal function declines progressively. To understand the mechanisms that contribute to renal disease, it is first necessary to understand the normal physiology of renal function. Thus in the following chapters in this section of the book, various aspects of renal function are considered. Both peritoneal dialysis and hemodialysis, as their names suggest, rely on the ability to remove small dialyzable molecules from the blood-including metabolic waste products normally removed by intact kidneys-via diffusion across a selectively permeable membrane into a solution lacking these substances, thereby mitigating both their accumulation and associated adverse health effects. In addition, dialysis helps reestablish both fluid and electrolyte balance via removal of excess fluid, correction of acid-base changes, and normalization of plasma electrolyte concentrations). In peritoneal dialysis, the peritoneal membrane lining the abdominal cavity acts as a dialyzing membrane. Several liters of a defined dialysis solution are typically introduced into the abdominal cavity, and small molecules in blood diffuse across the peritoneal membrane into the solution, which can then be iteratively removed, discarded, and replaced. Patients who are candidates for renal transplantation are often treated with dialysis until an appropriate donor kidney can be procured. Functional Anatomy of the Kidneys Structure and function are closely linked in the kidneys. Consequently an appreciation of the gross anatomical and histological features of the kidneys is a prerequisite for understanding their functions. Gross Anatomy the kidneys are paired organs that lie on the posterior wall of the abdomen behind the peritoneum on either side of the vertebral column. In an adult human, each kidney weighs between 115 and 170 g and is approximately 11 cm long, 6 cm wide, and 3 cm thick. The medial side of each kidney contains an indentation through which pass the renal artery and vein, nerves, and pelvis. If a kidney were cut in half, two regions would be evident: an outer region called the cortex and an inner region called the medulla. The cortex and medulla are composed of nephrons (the functional units of the kidney), blood vessels, lymphatics, and nerves. The base of each pyramid originates at the corticomedullary border, and the apex terminates in a papilla, which lies within a minor calyx. The numerous minor calyces expand into two or three open-ended pouches, the major calyces. The pelvis represents the upper expanded region of the ureter, which carries urine from the pelvis to the urinary bladder. The walls of the calyces, pelvis, and ureters contain smooth muscle that contracts to propel the urine toward the urinary bladder. The glomerular capillaries come together to form the efferent arteriole, which leads into a second capillary network, the peritubular capillaries, which supply blood to the nephron. The vessels of the venous system run parallel to the arterial vessels and progressively form the interlobular vein, arcuate vein, interlobar vein, and renal vein, which courses beside the ureter. The proximal tubule exits this structure and initially forms several coils, followed by a straight piece that descends toward the medulla. The next segment is the loop of Henle, which is composed of a the organization of the nephron is actually more complicated than presented here. However, for simplicity and clarity of presentation in subsequent chapters, the nephron is divided into five segments. However, again for simplicity, we consider the collecting duct system part of the nephron. Near the end of the thick ascending limb, the nephron passes between the afferent and efferent arterioles of the same nephron. The distal tubule begins a short distance beyond the macula densa and extends to the point in the cortex where two or more nephrons join to form a cortical collecting duct. The cortical collecting duct enters the medulla and becomes the outer medullary collecting duct and then the inner medullary collecting duct. Proximal tubule cells have an extensively amplified apical membrane (the ultrafiltrate or urine side of the cell) called the brush border, which is present only in the proximal tubule. The basolateral membrane (the interstitial or blood side of the cell) is highly invaginated. In contrast, the descending and ascending thin limbs of the loop of Henle have poorly developed apical and basolateral surfaces and few mitochondria. The cells of the thick ascending limb and the distal tubule have abundant mitochondria and extensive infoldings of the basolateral membrane. The collecting duct is composed of two cell types: principal cells and intercalated cells. Principal cells play an important role in reabsorption of NaCl (see Chapters 34 and 35) and secretion of K+ (see Chapter 36). The final segment of the nephron, the inner medullary collecting duct, is composed of inner medullary collecting duct cells, which have poorly developed apical and basolateral surfaces and few mitochondria. As described in more detail in Chapter 36, increased flow of tubule fluid in the collecting duct is a strong stimulus for secretion of K+. The increase in [Ca++] activates K+ channels in the apical plasma membrane, which enhances secretion of K+ from the cell into the tubule fluid. The corresponding loops of Henle are short, and associated efferent arterioles branch into peritubular capillaries that surround its associated nephron segments as well as adjacent nephrons. This capillary network conveys oxygen and important nutrients to the nephron segments in the cortex, delivers substances to individual nephron segments for secretion. When compared with superficial nephrons, juxtamedullary nephrons differ anatomically in two important ways: the loop of Henle is longer and extends deeper into the medulla, and the efferent arteriole forms not only a network of peritubular capillaries but also a series of accompanying vascular loops called the vasa recta. The endothelial cells of glomerular capillaries are covered by a basement membrane surrounded by podocytes. The filtration barrier is composed of three layers: the endothelium, basement membrane, and foot processes of the podocytes. Note the filtration slit diaphragm bridging the floor of the filtration slits (arrows). B, Scanning electron micrograph of the inner surface (blood side) of a glomerular capillary. In addition to their role as a barrier to filtration, the endothelial cells synthesize a number of vasoactive substances. Because both the basement membrane and filtration slits contain negatively charged glycoproteins, some proteins are held back. Mesangial cells, which possess many properties of smooth muscle cells, provide structural support for the glomerular capillaries, secrete extracellular matrix, exhibit phagocytic activity by removing macromolecules from the mesangium, and secrete prostaglandins and proinflammatory cytokines. Mesangial cells located outside the glomerulus (between the afferent and efferent arterioles) are called extraglomerular mesangial cells.
Topamax 200mg with mastercard
The stretch reflex is crucial for the maintenance of posture and helps overcome unexpected impediments during a voluntary movement treatment 2015 generic 100mg topamax free shipping. Changes in the stretch reflex are involved in actions commanded by the brain, and pathological alterations in this reflex are important signs of neurological disease. The tonic stretch reflex occurs in response to a slower or steady stretch applied to the muscle. Muscle spindles are found in almost all skeletal muscles and are particularly concentrated in muscles that exert fine motor control. Thus this reflex circuit essentially is a universal mechanism for helping govern muscle activity. The innervated part of the muscle spindle is encased in a connective tissue capsule. Muscle spindles lie between regular muscle fibers and are typically located near the tendinous insertion of the muscle. The ends of the spindle are attached to the connective tissue within the muscle (endomysium). The key point is that muscle spindles are connected in parallel with the regular muscle fibers and thus are able to sense changes in the length of the muscle. The muscle fibers within the spindle are called intrafusal fibers, to distinguish them from the regular or extrafusal fibers that make up the bulk of the muscle. Individual intrafusal fibers are much narrower than extrafusal fibers and do not run the length of the muscle. Thus they are too weak to contribute significantly to muscle tension or to cause changes in the overall length of the muscle directly by their contraction. Skeletal muscles contain sensory receptors embedded within the muscle(spindles)andwithintheirtendons(Golgitendonorgans). The neural innervation of an intrafusal fiber differs significantly from that of an extrafusal fiber, which is innervated by a single motor neuron. Intrafusal fibers are multiply innervated and receive both sensory and motor innervation. A group Ia afferent fiber forms a spiral-shaped termination, referred to as a primary ending, on each of the intrafusal muscle fibers in the spindle. Thus primary endings are found on both types of nuclear bag fibers and on nuclear chain fibers. The primary and secondary endings have mechanosensitive channels that are sensitive to the level of tension on the intrafusal muscle fiber. Dynamic motor axons end on nuclear bag1 fibers, and static motor axons end on nuclear chain and bag2 fibers. Muscle Spindles Detect Changes in Muscle Length Muscle spindles respond to changes in muscle length because they lie in parallel with the extrafusal fibers and therefore are also stretched or shortened along with the extrafusal fibers. The nonselective cation channel Piezo2 has been identified as the principal transduction channel that allows spindle sensory afferent fibers to sense changes in mechanical stress that occur when a muscle changes length. Group Ia fibers show this same static-type response, and thus under steady-state conditions. While muscle length is changing, however, group Ia firing also reflects the rate of stretch or shortening that the muscle is undergoing. Its activity overshoots during muscle stretch and undershoots (and possibly ceases) during muscle shortening. In particular, the tap profile is what occurs when a reflex hammer is used to hit the muscle tendon and thereby cause a brief stretching of the attached muscle. Thus the functionality of reflex arcs involving group Ia afferent fibers is what is being assessed when a reflex hammer is used to tap on tendons. Up to this point, we have described only how muscle spindles behave when there are no changes in motor neuron activity. The efferent innervation of muscle spindles is extremely important, however, because it determines the sensitivity of muscle spindles to stretch. C, Coactivation of and motor neurons causes shortening of both extrafusal and intrafusalfibers. If this happens, the muscle spindle afferent fiber may stop discharging and become insensitive to further decreases in muscle length. However, the unloading of the spindle can be prevented if and motor neurons are stimulated simultaneously. Note that only the two polar regions of the intrafusal muscle contract; the equatorial region, where the nuclei are located, does not contract because it has little contractile protein. Nevertheless, when the polar regions contract, the equatorial region elongates and regains its sensitivity. Conversely, when a muscle relaxes (motor neuron activity drops) and thus elongates (if its ends are being pulled), a concurrent decrease in motor neuron activity allows the intrafusal fibers to relax (and thus elongate) as well and thereby prevent the tension on the central portion of the intrafusal fiber from reaching a level at which firing of the afferent fibers is saturated. Thus the motor neuron system allows the muscle spindle to operate over a wide range of muscle lengths while retaining high sensitivity to small changes in length. For voluntary movements, descending motor commands from the brain in fact typically activate and motor neurons simultaneously, presumably to maintain spindle sensitivity as just described. Second, if the spindle were to become unloaded during the movement, this would oppose the intended movement by decreasing the excitatory drive, via the group Ia reflex arc (see next section), to the motor neurons driving the agonist muscles. Dynamic motor axons end on nuclear bag1 fibers, and static motor axons synapse on nuclear chain and bag2 fibers. Descending pathways can preferentially influence dynamic or static motor neurons and thereby alter the nature of reflex activity in the spinal cord and also, presumably, the functioning of the muscle spindle during voluntary movements. A rapid stretch of the rectus femoris muscle strongly activates the group Ia fibers of the muscle spindles, which then convey this signal into the spinal cord. In the spinal cord, each group Ia afferent fiber branches many times to form excitatory synapses directly (monosynaptically) on virtually all motor neurons that supply the same (also known as the homonymous) muscle and with many motor neurons that innervate synergists, such as the vastus intermedius muscle in this case, which also acts to extend the leg at the knee. If the excitation is powerful enough, the motor neurons discharge and cause a contraction of the muscle. Note that the group Ia fibers do not contact the motor neurons, possibly to avoid a positive-feedback loop situation. This selective targeting of motor neurons is exceptional in that most other reflex and descending pathways target both and motor neurons. They end on motor neurons that innervate the antagonist muscles-in this case, the hamstring muscles, including the semitendinosus muscle-which act to flex the knee. Other branches of the group Ia afferent fibers synapse with yet other neurons that originate ascending pathways that provide various parts of the brain (particularly the cerebellum and cerebral cortex) with information about the state of the muscle. The organization of the stretch reflex arc guarantees that one set of motor neurons is activated and the opposing set is inhibited. Although many reflexes involve such reciprocal innervation, this type of innervation is not the only possible organization of a motor control system; descending motor pathways can override such patterns. The stretch reflex is quite powerful, in large part because of its monosynaptic nature. That is, each group Ia fiber contacts virtually all homonymous motor neurons, and each such motor neuron receives input from every spindle in that muscle. Although its monosynaptic nature makes the group Ia reflex rapid and powerful, it also means that there is relatively little opportunity for direct control of the tonic stretch reflex can be elicited by passive bending of a joint. For example, if the knee of a soldier standing at attention begins to flex because of fatigue, the quadriceps muscle is stretched, a tonic stretch reflex is elicited, and the quadriceps contracts more, thereby opposing the flexion and restoring the posture. The foregoing discussion suggests that stretch reflexes can act like a negative-feedback system to control muscle length. By following the stretch reflex arc, it is possible to see that changes in its activity act to oppose changes in muscle length from a particular equilibrium point. Similarly, passive shortening of the muscle unloads the spindles and leads to a decrease in the excitatory drive to the motor neurons and thus relaxation of the muscle. It is partly because the motor neurons are coactivated during a movement and thereby shift the equilibrium point of the spindle and partly because the gain or strength of the reflex is low enough that other input to the motor neuron can override the stretch reflex. Inverse Myotatic or Group Ib Reflex the inverse myotatic reflex acts to oppose changes in the level of force in the muscle. Just as the stretch reflex can be thought of as a feedback system to regulate muscle length, the inverse myotatic, or group Ib, reflex can be thought of as a feedback system to help maintain force levels in a muscle. The arc starts with the Golgi tendon organ receptor, which senses the tension in the muscle.
Buy topamax 200mg with mastercard
Rods adapt to darkness slowly as their rhodopsin levels are restored symptoms of flu proven 100mg topamax, and, indeed, it may take more than 30 minutes for the retina to become fully dark-adapted. Thus within 10 minutes in a dark room, rod vision is more sensitive than cone vision and becomes the main system for seeing. In sum, in the dark-adapted state, primarily rod vision is operative, and thus visual acuity is low and colors are not distinguished (this is called scotopic vision). There is an intermediate range of light levels at which rod and cones are both functional (mesopic vision). More indirect pathways that provide for intraretinal signal processing involve photoreceptors, bipolar cells, amacrine cells, and ganglion cells, as well as horizontal cells to provide lateral interactions between adjacent pathways. Color Vision the visual pigments in the cone outer segments contain different opsins. As a result of these differences, the three types of cones absorb light best at different wavelengths. According to the trichromacy theory, the differences in absorption efficiency of the cone visual pigments are presumed to account for color vision because a suitable mixture of three colors can produce any other color. Two or three of the cone pigments may absorb a particular wavelength of light, but the amount absorbed by each differs according to its efficiency at that wavelength. If the intensity of the light is increased (or decreased), all will absorb more (or less), but the ratio of absorption among them will remain constant. Consequently, there must be a neural mechanism to compare the absorption of light of different wavelengths by the different types of cones for the visual system to distinguish different colors. The presence of three kinds decreases the ambiguity in distinguishing colors when all three absorb light, and it ensures that at least two types of cones will absorb most wavelengths of visible light. The opponent process theory is based on observations that certain pairs of colors seem to activate opposing neural processes. For example, if a gray area is surrounded by a green ring, the gray area appears to acquire a reddish color. These observations are supported by findings that neurons activated by green wavelengths are inhibited by red wavelengths. Similarly, neurons excited by blue wavelengths may be inhibited by yellow wavelengths. Contrasts in Rod and Cone Pathway Functions Rod and cone pathways have several important functional differences in their phototransduction mechanisms and their retinal circuitry. As described previously, rods have more visual pigment and a better signal amplification system than cones do, and there are many more rods than cones. Thus rods function better in dim light (scotopic vision), and loss of rod function results in night blindness. In addition, all rods contain the same visual pigment, so they cannot signal color differences. Furthermore, many rods converge onto individual bipolar cells and the results are very large receptive fields and low spatial resolution. Finally, in bright light, most rhodopsin is bleached, so that rods no longer function under photopic conditions. Cones have a higher threshold to light and thus are not activated in dim light after dark adaptation. They provide high-resolution vision because only a few cones converge onto individual bipolar cells in cone pathways. Moreover, no convergence occurs in the fovea, where the cones make one-to-one connections to bipolar cells. As a result of the reduced convergence, cone pathways have very small receptive fields and can resolve stimuli that originate from sources very close to each other. Photoreceptors (R) synapse on the dendrites of bipolar cells (B) and horizontal cells (H) in the outer plexiformlayer. Loss of cone function results in functional blindness; rod vision is not sufficient for normal visual requirements. Synaptic Interactions and Receptive Field Organization the receptive field of an individual photoreceptor is circular. Light in the receptive field hyperpolarizes the photoreceptor cell and cause it to release less neurotransmitter. The receptive fields of photoreceptors and retinal interneurons determine the receptive fields of the retinal ganglion cells onto which their activity converges. The characteristics of the receptive fields of retinal ganglion cells constitute an important step in visual information processing because all the information about visual events that is conveyed to the brain is contained in ganglion cell activity. Both are described as having a center-surround organization in which the light that strikes the central region of the receptive field either excites or inhibits the cell, whereas the light that strikes a region that surrounds the central portion has the converse effect. The center response of a bipolar cell receptive field is due to only the photoreceptors that directly synapse with the bipolar cell. Photoreceptor cells respond to light with hyperpolarization and a decrease in glutamate release and respond to the removal of light with depolarization and increased glutamate release. This implies that the difference in the center responses of "on" and "off " bipolar cells lies in their response to glutamate. In contrast, on-center bipolar cells have metabotropic glutamate receptors that close their channels in response to glutamate. They are depolarized by light on the center of their receptive field, because the reduced release of glutamate by the photoreceptors results in more open metabotropic channels. Thus on-center bipolar cells are excited by light stimulation of the center of their receptive fields. The antagonistic surround response of bipolar cells is due to photoreceptors that surround those that synapse directly on them. These photoreceptors (which also connect directly with their own bipolar cells) synapse with horizontal cells that participate in complex triadic synapses with many photoreceptors and bipolar cells. The reason for this is that horizontal cells are depolarized by glutamate released from photoreceptors and thus, like "off" bipolar cells, are hyperpolarized in the light. Moreover, because they are electrically coupled to each other by gap junctions, they have very large receptive fields. Thus when darkness surrounds central illumination, there is increased excitation of on-center bipolar cells. Bipolar cells may not respond to large or diffuse areas of illumination, covering both the receptors that are responsible for the center response and those that cause the surround response because of their opposing actions. Thus bipolar cells may not signal changes in the intensity of light that strikes a large area of the retina. On the other hand, a small spot of light moving across the receptive field may sequentially and dramatically alter the activity of the bipolar cell as the light crosses the receptive field from the surround portion to the center and then back again to the surround portion. This demonstrates that bipolar cells respond best to the local contrast of stimuli and function as contrast detectors. Amacrine cells receive input from different combinations of on-center and off-center bipolar cells. There are many different types of amacrine cells, and they may use at least eight different neurotransmitters. Accordingly, the contributions of amacrine cells to visual processing are complex. When amacrine cell input dominates, the receptive fields of ganglion cells tend to be diffuse, and they are either excitatory or inhibitory. Hence, modulation of transmitter release by changes in transmembrane potential and the resulting postsynaptic potentials are sufficient for most of the activity in retinal circuits, and action potentials are not required except for ganglion cells and some amacrine cells, which generate action potentials. It is unclear why amacrine cells have action potentials, but ganglion cells must generate them to transmit information over the relatively long distance from the retina to the brain. P, M, and W Cells Experiments have shown that in primates, retinal ganglion cells can be subdivided into three general types called P cells, M cells, and W cells. P and M cells have center-surround receptive fields; hence, they are presumably controlled by bipolar cells. They are probably influenced chiefly through amacrine cell pathways, but less is known about them than about M and P cells. Several of the physiological differences among these cell types correspond to morphological differences (Table 8. For example, P cells have small receptive fields (which corresponds to smaller dendritic trees) and more slowly conducting axons than M cells do. In addition, P cells show a linear response in their receptive field; that is, they respond with a sustained, tonic discharge of action potentials in response to maintained light but do not signal shifts in the pattern of illumination as long as the overall level of illumination is constant. Because there are blue, green, and red cones, many combinations of color properties are possible, but in fact P cells have been shown to have opposing responses only to red and green or only to blue and yellow (a combination of red and green).