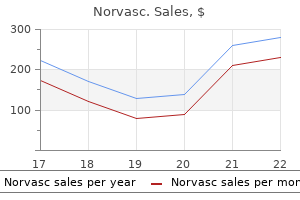
Effective 2.5 mg norvasc
Primary pulmonary meningioma is an uncommon primary tumor of the lung that is typically a single isolated lesion blood pressure medication that doesn't cause cough order generic norvasc line. The possibility should be considered if a limited biopsy shows meningothelial cells. The differential diagnosis with neuroendocrine proliferations of the lung, namely tumorlets and carcinoids, is based on the expression of cytokeratin, chromogranin A, and synaptophysin in neuroendocrine lesions. Paraganglioma distinguishes itself by the positivity for neuroendocrine markers coupled with negativity for cytokeratin and presence of intermingled stellate S100+ spindle cells. The onset of shortness of breath is typically preceded by a prodrome of viral-like illness with cough and fever and respiratory failure within days or weeks. Patients with sepsis, advanced age, and multiorgan failure have a worse prognosis. Histologic Features the early exudative phases show necrosis of pneumocytes, endothelial cells, intra-alveolar fibrinous exudates, and formation of hyaline membranes. Diffuse interstitial fibroblastic proliferation associated with edematous myxomatous stroma and collapse of alveolar parenchyma is seen. Organizing phase shows organization of hyaline membranes with formation of airway space granulation tissue. In such cases, it is important to perform immunostains for viral antigens and special stains to rule out other infectious organisms. Most patients are between the ages of 50 and 70 years, with an average age of onset in the sixth decade. Traction bronchiectasis and honeycomb changes can also be seen in similar distribution. However, often in 50% of cases, there may be other nonspecific findings, such as groundglass opacities, upper lobe distribution, and absence of honeycomb changes, thus necessitating the need for a surgical lung biopsy. These patients are at increased risk for lung cancer, pulmonary hypertension/cor pulmonale, respiratory failure secondary to progressive fibrosis, and increased pulmonary infections. Median survival rates vary in the average range of 2 to 3 years in large series but approaching 4 to 6 years in few. The presence of underlying collagen vascular disease is associated with longer survival. Histologic Features Heterogenous, patchwork pattern of lung fibrosis whereby scarred areas of lung are present adjacent to normal lung parenchyma resulting in spatial variability is a characteristic 1009 feature. Clear evidence of chronic scarring and architectural destruction in the form of microscopic honeycombing and traction bronchiectasis. The patchy pattern of interstitial fibrosis also results in temporal heterogeneity demonstrated by the presence of active fibroblastic foci representing "younger" foci of active ongoing disease compared with the more abundant "older" mature scarring areas. Fibrosis has a typical peripheral/subpleural accentuation and paraseptal distribution. End-stage honeycomb changes are characterized by enlarged airspaces with thickened fibrotic walls, lined by bronchiolar epithelium and inspissated mucus in the lumen of these spaces. Squamous metaplasia of the epithelial lining and hyperplasia of the smooth muscle are also common associated findings. Lymphoid follicles with germinal centers in the fibrotic lung and pleura tend to be prominent in cases associated with collagen vascular diseases such as rheumatoid arthritis. Vascular changes are also common in the scarred areas, including intimal proliferation and medial thickening of muscular pulmonary arteries. Pulmonary function tests demonstrate restriction and a low diffusing capacity for carbon monoxide. High-resolution computed tomography abnormalities include predominantly lower lobe subpleural reticular changes, traction bronchiectasis, and ground-glass opacities; honeycombing is rarely seen. It is commonly seen in middle-aged to older adults and is often preceded by a flu-like illness. Persistent nonproductive cough and shortness of breath are the usual presenting symptoms. It is a common histopathologic pattern seen in patients with subacute lung injury, which can be seen secondary to infections (bacterial or fungal), aspiration pneumonia, and drug reactions and can be seen as a minor component of many other diseases, such as hypersensitivity pneumonitis, collagen vascular disease, and Wegener granulomatosis. The idiopathic cases of organizing pneumonia are referred to as cryptogenic organizing pneumonia. Most, but not all, patients respond rapidly to steroids, and in most cases, prognosis is excellent. Prognosis of patients with secondary organizing pneumonia depends on the underlying condition. Histologic Features Nodules of granulation tissue (fibroblasts in an edematous or myxoid stroma) in and around small airways with typical branching appearance. Mild to moderate interstitial inflammation composed of lymphocytes and plasma cells is typically seen. No significant architectural destruction, neutrophils, eosinophils, vasculitis, or granulomas are seen. Initial reports, originated in Japanese literature, were referred to as idiopathic pulmonary upper lobe fibrosis, also termed Amitani disease. This disease presents in adults with a median age of 57 years with no sex predilection. Approximately half of the patients experience recurrent infections or may have an autoimmune 1023 condition, whereas few may have familial interstitial lung disease and nonspecific autoantibodies. Most patients present with dyspnea on exertion and dry cough and have restrictive lung function tests. High-resolution computed tomography show subpleural reticular/nodular opacities, initially favoring the upper zones followed by pleural fibrosis and linear or wedge-shaped extension down secondary lobular septa. The middle and lower zones may not be affected initially, but progressive envelopment of these regions occurs with time. In more advanced cases, volume loss and traction bronchiectasis may be prominent features. Survival characteristics depend on the stage of the disease at presentation because some patients are diagnosed in a relatively asymptomatic phase. Histological Features Gross specimen description in the literature is limited, likely reflecting the paucity of recognized cases at autopsy or the rare cases of transplantation. Microscopic examination shows dense collagenous fibrosis with or without concomitant elastosis of the visceral pleura; encasement of the lung begins in the apical region, with progressive extension into the inferior zones. Dense subpleural bands of parenchymal fibroelastosis follow this same distribution. The pattern is histologically distinctive in that vague outlines of alveoli are preserved or even accentuated by elastotic proliferation, presumably within the alveolar septa. The enclosed airspaces are filled with collagenous fibrosis of variabl e densi ty. The inflammatory component within the fibrous regions is typically sparse and lymphocytic, occasionally with lymphoid 1024 follicles. In these settings, pulmonary histology may reflect general lung injury patterns, such as bronchiolitis or diffuse alveolar damage. However, some viruses cause characteristic cytopathic changes, including multinucleation, cytomegaly, and inclusions (nuclear and/or cytoplasmic). Cytoplasmic inclusions, usually basophilic, but occasionally may be more eosinophilic. Inclusions are seen in pneumocytes and endothelial cells; however, mesenchymal cells such as smooth muscle cells can also be involved. The interstitial pneumonitis is mild and lymphocytic, but severe lung injury with diffuse alveolar damage can also occur. Nuclear enlargement and multinucleation are characteristic, but nuclear inclusions, while present, can be harder to identify. Immunohistochemistry can be very useful in confirming the diagnosis in suspicious cases. Nodules can have central necrosis but are not granulomatous; they can become fibrotic and hyalinized or can calcify. It can result in upper respiratory infection, pneumonia with bronchiolitis, and conjunctivitis.
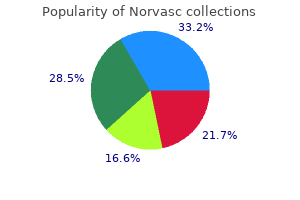
Discount norvasc 10mg fast delivery
The first steps in virus attachment are governed largely by the probability that a virus particle and a cell will collide pulse pressure compliance buy generic norvasc 10mg on line, and therefore by the concentrations of free particles and host cells. In this example, the poliovirus receptor is the determinant of poliovirus host range. However, production of the receptor in a particular cell type does not ensure that virus reproduction will occur. Some primate cell cultures produce the poliovirus receptor but cannot be infected. The restriction of viral reproduction in these cells is most probably due to a block in viral reproduction beyond the attachment step. Receptors can also be determinants of tissue tropism, the predilection of a virus to invade and reproduce in a particular cell type. For example, the sialic acid residues on membrane glycoproteins or glycolipids, which are receptors for influenza where A is attachment, t is time, and [V] and [H] are the concentrations of virus particles and host cells, respectively, and k is a constant that defines the rate of the reaction. It can be seen from this equation that if a mixture of viruses and cells is diluted after an adsorption period, subsequent binding of particles is greatly reduced. For example, a 100-fold dilution of the virus and cell mixture reduces the attachment rate 10,000-fold. Dilution can be used to prevent subsequent virus adsorption and hence to synchronize an infection. Many receptor molecules can move in the plasma membrane, leading to the formation of microdomains that differ in composition. Bound virus may therefore localize to specialized areas of the membrane such as lipid rafts, caveolae, or coated pits. Localization of virus particle-receptor complexes can also cause transmembrane signaling, changes in the cytoskeleton, and recruitment of clathrin. Identification of Receptors for Virus Particles the development of three crucial technologies in the past 30 years has enabled identification of many receptors for viruses. Production of monoclonal antibodies provided a powerful means of isolating and characterizing individual cell surface proteins. Hybridoma cell lines that secrete monoclonal antibodies that block virus attachment are Attachment and Entry 125 obtained after immunizing mice with intact cells. Such antibodies can be used to purify the receptor protein by affinity chromatography. Clones of such cells are recognized and selected, for example, by the binding of receptor-specific monoclonal antibodies. The receptor genes can then be isolated from these selected cells by using a third technology, molecular cloning. The power of these different technologies can lead to rapid progress: the receptor for a newly identified Middle Eastern respiratory syndrome coronavirus was identified just 4 months after the first description of the virus (Box 5. Although these technologies have led to the identification of many cell receptors for viruses, each method has associated uncertainties (Box 5. The availability of receptor genes has made it possible to investigate the details of receptor interaction with viruses by site-directed mutagenesis. Receptor proteins can be synthesized in heterologous systems and purified, and their properties can be studied in vitro, while animal cells producing altered receptor proteins can be used to test the effects of alterations on virus attachment. Because of their hydrophobic membrane-spanning domains, many of these cell surface proteins are relatively insoluble and difficult to work with. Three different strategies for identifying such rare receptor-expressing cells are outlined. Cells that make the correct receptor and become infected with such viruses can be distinguished by a color change, such as green in the case of green fluorescent protein. The fusion protein was produced in cells and incubated with lysates of cells known to be susceptible to the virus, and the resulting complexes were fractionated by native polyacrylamide gel electrophoresis. This polypeptide was excised from the polyacrylamide gel, and its amino acid sequence was determined by mass spectrometric analysis, identifying it as dipeptidyl peptidase 4. That a single protein was identified by this procedure is remarkable: typically, this approach identifies many nonspecific binding proteins. Soluble extracellular protein domains (with the virus binding sites) have been essential for structural studies of receptor-virus interactions. Receptor genes have also been used to produce transgenic mice that synthesize receptor proteins. Such transgenic animals can serve as useful models in the study of human viral diseases. Virus-Receptor Interactions Animal viruses have multiple receptor-binding sites on their surfaces. Of necessity, one or more of the capsid proteins of nonenveloped viruses specifically interact with the cell receptor. Receptor-binding sites for enveloped viruses are provided by oligomeric type 1 integral membrane glycoproteins encoded by the viral genome that have been incorporated into the cell-derived membranes of virus particles. Although the details vary among viruses, most virus-receptor interactions follow one of several mechanisms illustrated by the best-studied examples described below. Nonenveloped Viruses Bind via the Capsid Surface or Projections Attachment via surface features: canyons and loops. Members of the enterovirus genus of the Picornaviridae include human polioviruses, coxsackieviruses, echoviruses, enteroviruses, and rhinoviruses. It was known that mouse cells cannot be infected with poliovirus, because they do not produce the receptor. Consequently, it was possible to develop a small-animal model for poliomyelitis by producing transgenic mice that synthesize this receptor. Similar approaches have subsequently led to animal models for viral diseases caused by measles virus and echoviruses. Rhinoviruses multiply primarily in the upper respiratory tract and are responsible for causing up to 50% of all common colds. Over 150 rhinovirus genotypes have been identified and classified on the basis of genome sequence into three species, A, B, and C. A monoclonal antibody that blocks virus attachment might recognize not the receptor but a closely associated membrane protein. However, the encoded protein might not be a receptor but may modify another cellular protein so that it can bind virus particles. For some viruses, synthesis of the receptor on cells leads to binding but not infection. In such cases a coreceptor is required, either for internalization or for membrane fusion. The cell surface receptor bound by most A and B species rhinoviruses was identified by using a monoclonal antibody that blocks rhinovirus infection and that recognizes a cell surface protein. This monoclonal antibody was used to isolate a 95-kDa cell surface glycoprotein by affinity chromatography. Amino acid sequence analysis of the purified protein, which bound to rhinovirus in vitro, identified it as the integral membrane protein intercellular adhesion molecule 1 (Icam-1). Cell receptors for other rhinoviruses are the low-density lipoprotein receptor and cadherin-related family member 3. The canyons in the capsids of some rhinoviruses and enteroviruses are the sites of interaction with cell surface receptors. Amino acids that line the canyons are more highly conserved than any others on the viral surface, and their substitution can alter the affinity of binding to cells. Although canyons are present in the capsid of rhinovirus type 2, they are not the binding sites for the receptor, low-density lipoprotein receptor. Sequence and structural comparisons have revealed why different rhinovirus serotypes bind distinct receptors. The results of competition experiments indicated that members of two different virus families, group B coxsackieviruses and most human adenoviruses, share a cell receptor. This receptor is a 46-kDa member of the Ig superfamily called Car (coxsackievirus and adenovirus receptor). Interaction with a coreceptor, the v integrin v 3 or v 5, is required for uptake of the capsid into the cell by receptor-mediated endocytosis. An exception is adenovirus type 9, which can infect hematopoietic cells after binding directly to v integrins. The fibers are composed of homotrimers of the adenovirus fiber protein and are anchored in the pentameric penton base; both proteins have roles to play in virus attachment and uptake. For many adenovirus serotypes, attachment via the fibers is necessary but not sufficient for infection.
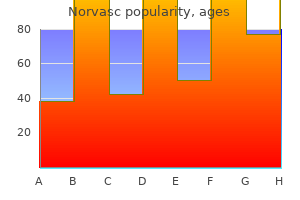
Discount norvasc 10 mg with amex
Bois Natasha Rekhtman Pulmonary chondromas are rare benign tumors of mature and immature cartilaginous tissue arrhythmia quality services purchase norvasc 10mg fast delivery. Myxoid degeneration, infarct-type necrosis, and calcification (often in the form of osseous metaplasia) are frequently encountered. The pathologic diagnosis is based on histologic features, and immunohistochemistry is generally noncontributory. The differential diagnosis includes primarily pulmonary hamartoma and primary or metastatic chondrosarcoma. The former has entrapped epithelial-lined spaces, lacks a fibrous pseudocapsule, and by definition has more than one mesenchymal component. The latter can be distinguished by higher cellularity and the presence of cytologic atypia. On low-power magnification, it appears as a rounded circumscribed tumor with patchy areas of hypocellular dense collagenized stroma, numerous thin-walled vessels that are often dilated and branched (so-called hemangiopericytoma-like), and often several areas of entrapped pulmonary epithelium in cleft-like spaces. The lesional cells are haphazardly arranged, with bland plump oval to spindleshaped nuclei with inconspicuous nucleoli, open chromatin, and scant to inapparent clear cytoplasm. As the name implies, the tumor matrix consists of abundant dense ropy hyalinized collagen, which is interwoven between tumor cells. The differential diagnosis includes synovial sarcoma, desmoid-type fibromatosis, spindle-cell carcinoid tumor, and primary or metastatic spindle-cell lesions of neural or smooth muscle origin. The recognition of relatively characteristic histologic features along with a concise immunohistochemical panel usually allow for relatively clear distinction between these entities. Although they are considered benign because of their inability to metastasize, they are locally aggressive tumors. On low-power magnification, they appear as a relatively uniform and hypocellular lesion with irregular and infiltrative borders. The tumor cells are medium-sized filiform to plump fibroblasts with characteristic pale nuclei, often in parallel orientation, embedded in a variably dense but usually loose fibrillar collagenous stroma with scattered arterioles. Most cases arise sporadically, with antecedent trauma/surgery and female sex (particularly pregnancy) as risk factors, whereas a minority of cases occur in the setting of Gardner syndrome. The differential diagnosis also includes low-grade fibromyxoid sarcoma, which can be morphologically similar but 546 generally has prominent myxoid areas and is negative for -catenin. They are generally isolated lesions discovered incidentally in resection specimens, however, occasionally are multiple and can produce a diffuse micronodular pattern with radiologic mosaic attenuation, such as in the setting of diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. Although both neuroendocrine cell hyperplasia and carcinoid tumorlets are believed to originate from naturally occurring bronchial neuroendocrine cells (Kulchitsky cells), the latter extend beyond the basement membrane of the bronchiolar epithelium, whereas the former do not. Other than overall size, they are morphologically identical to typical carcinoid tumors of the lung, and thus the same spectrum of histologic (trabecular, nested, pseudoglandular) and cytomorphologic (round, spindle, oncocytic) features can be observed. They are generally (but not universally) positive for broad-spectrum cytokeratins. Because of their small size and occasional peripheral location, they can be confused with meningothelioid nodules. When tumorlets are multiple, metastatic neuroendocrine tumor should be considered, and careful examination of tumor location (peribronchiolar vs intravascular) 549 and clinicopathologic correlation is necessary. As their name implies, they are small (generally <5 mm), but otherwise morphologically and immunohistochemically identical to intracranial and extracranial meningiomas. They are generally incidentally identified in resection specimens, often adjacent to interlobular septa and/or pulmonary venules, proliferating in small whorls within and expanding the interstitium, resulting in a vaguely stellate lesion. Cytologically the lesional cells are oval to spindle-shaped with dense eosinophilic cytoplasm, indistinct cell borders, finely granular chromatin, and indistinct nucleoli. In contrast to meningiomas, minute meningothelioid nodules are considered reactive lesions, although there is some evidence to suggest that they may harbor neoplastic molecular driver aberrations in the setting of diffuse bilateral disease ("diffuse pulmonary meningotheliomatosis"). The differential diagnosis is relatively limited and includes carcinoid tumorlets, which are peribronchiolar and positive for chromogranin and synaptophysin, and small vascular proliferations such as plexiform lesions. Rarely, diffuse pulmonary meningotheliomatosis can simulate metastasis of 554 differentiated carcinoma; however, immunohistochemical workup and close clinical and radiologic correlation can generally resolve this diagnostic dilemma. Immunohistochemical and molecular studies have shown that both cell types are clonal and believed to originate from a common primitive respiratory epithelial precursor. They occur more commonly in women and are generally peripheral and solitary, although multifocal cases have been reported. Tumor borders are well circumscribed and, as the name implies, variable amounts of dense tumoral fibrosis can be seen even at low power. Often several architectural patterns occur within the same lesion, including papilliform, sclerotic, solid, and hemorrhagic. The lesional cells are often best viewed at the periphery of the lesion, as stromal fibrosis frequently obscures them centrally. The stromal cells are round, with a moderate amount of pale eosinophilic cytoplasm, generally vesicular chromatin, and small visible nucleoli (occasional nuclear grooves can be seen). The surface cells are cuboidal, tend to appear slightly smaller than the round cells, with denser eosinophilic cytoplasm and more condensed chromatin. Diagnosis on small biopsy specimens can be challenging and the differential diagnosis includes pulmonary adenocarcinoma, metastatic papillary thyroid 558 or renal cell carcinoma, papillary adenoma, hemangioma, and Langerhans cell histiocytosis. The key to the correct diagnosis is the lack of significant cytologic atypia of the surface cells as well as recognition and immunohistochemical characterization of the stromal round cells. Epithelial cells are present singly and in rosettes (inset), with occasional intranuclear pseudoinclusions (arrow). It is believed to be a neoplasm; however, it remains unclear if only one or both of the components are neoplastic, and studies to date suggest that it is unlikely that the two cell populations originate from a common progenitor. On low-power magnification, the tumor is characterized by well-circumscribed borders, with multiple variably sized cystic spaces, which are lined by plump epithelial cells (often discontinuous) and are often filled with amphophilic granular debris and rare macrophages. The intratumoral and peritumoral interstitium contains plump ovoid to spindle-shaped fibroblast-like cells in a variably myxoid matrix with occasional mast cells and/or lymphocytes and plasma cells. The morphologic differential diagnosis includes lymphangioma, atypical adenomatous hyperplasia, sclerosing pneumocytoma, and pulmonary hamartoma. Recognition of the prominent cystic spaces, biphasic morphology without differentiated mesenchymal elements, and lack of cytologic atypia, generally allows for distinction from these entities. They are generally peripherally located and solitary, and tend to occur more commonly in males. On low-power magnification, they appear as multiple simple and focally branching papillary structures that compress adjacent and intratumoral interstitium, occasionally giving rise to dilated-appearing alveolar airspaces filled with papillae. The lining of the papilla consists of a continuous single row of cuboidal to columnar epithelial cells with pneumocytic differentiation. The main entities in the differential diagnosis are primary or metastatic papillary adenocarcinoma and sclerosing pneumocytoma. As their origin implies, they tend to occur centrally, in a peribronchial or endobronchial location, and frequently manifest with clinical signs and symptoms of obstruction. The tumor typically has well-circumscribed pushing borders and consists of multiple glandular spaces lined by uniform nonciliated columnar cells with basally oriented nuclei and abundant clear intracytoplasmic mucin. The intervening stroma is generally compressed and contains scattered spindle cells and collagen. The stromal cells demonstrate focal positivity for broad-spectrum cytokeratin and S100, suggesting a myoepithelial component.
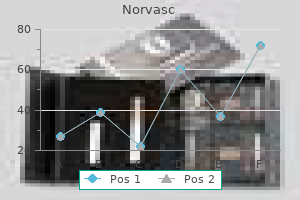
Order generic norvasc canada
Viral glycoproteins may be proteolytically processed in the Golgi network pulse pressure emt purchase cheapest norvasc and norvasc, a reaction essential for the formation of infectious particles. Viral components are sorted to specialized surfaces in polarized cells, including epithelial cells and neurons. The matrix or tegument proteins of enveloped viruses, which lie between the inner surface of the membrane and the capsid, do not enter the secretory pathway, but are synthesized in the cytoplasm and directed to membrane assembly sites by specific signals. The envelopes of a variety of viruses are acquired from internal membranes of the infected cell, rather than from the plasma membrane. Progeny genomes, structural proteins, and enzymes of virus particles must be concentrated at the intracellular site at which assembly takes place. The movement of viral components requires transport systems and a considerable expenditure of energy, supplied by the host cell. The particles of many viruses that reproduce in animal cells include a lipid envelope derived from a host cell membrane host cell, and therefore assembly takes place at a cell membrane. Distributions of high-molecular-weight reactants and products are rarely controlled by concentration gradients and diffusion, as they are in vitro. Indeed, the inside of a cell is so tightly packed with organelles and cytoskeletal structures (panel A in the figure) that it is simply inappropriate to think of the contents of the cytoplasm, the nucleus, or organelle lumens as "gels" or "suspensions. Short-distance movement across membranes or in and out of capsids is measured in angstroms to nanometers and is accomplished primarily via protein channels. Long-distance movement of proteins, viral particles or their components, and organelles inside cells is measured in micrometers to meters. Such movement invariably requires energy and is mediated by molecular motors moving on cytoskeletal tracks; myosins move cargo on actin fibers, while dynein and kinesin move cargo on microtubules. This model was constructed by combining the results of several complementary approaches: quantitative immunoblotting and quantitative mass spectrometry to measure the number of molecules of each protein; electron microscopy to determine the number, size, and positions of organelles; and super-resolution fluorescence microscopy to localize the proteins. Previously determined molecular structures of the proteins and their interactions were also used. Eukaryotic cells are therefore constantly engaged in transport of macromolecules among their compartments via intracellular trafficking systems. Indeed, the advances in our understanding of cellular trafficking mechanisms can be traced to initial studies of viral membrane or nuclear proteins. In the following sections, the cellular transport pathways required during viral reproduction are described in the context of the site at which virion assembly takes place. In these cases, only a subset of viral structural proteins must be imported into the nucleus. The nucleus and major membrane-bound compartments of the cytoplasm, as well as components of the cytoskeleton, are illustrated schematically and not to scale. They engage with the cytoplasmic face of the nuclear pore complex and are translocated into the nucleus by the protein import machinery of the host cell. As far as we know, all viral structural proteins that enter the nucleus do so via the normal cellular pathways of nuclear protein import. These same pathways are responsible for import of both viral genomes (or nucleoproteins) and viral nonstructural proteins that function in the nucleus early in the infectious cycle (Chapter 5). Import of Viral Proteins for Assembly the primary sequences of many such viral proteins destined for nuclear import contain putative nuclear localization sequences, which are characterized by clusters of basic amino acids. A typical mammalian cell contains on the order of 3,000 to 4,000 nuclear pore complexes, each with a very high translocation capacity, with 103 translocation events/s. However, nuclear import also depends on the limited supply of soluble transport proteins. As large quantities of viral structural proteins must enter the nucleus prior to assembly, there is potential for competition among viral and cellular proteins for access to receptors or the nuclear pore complex proteins that mediate transport. They travel via transport vesicles to and through the Golgi apparatus and then to the plasma membrane. The internal proteins of the particle (blue) and the genome (green) are also directed to plasma membrane sites of assembly. The proteins of viruses that do not induce inhibition of cellular protein synthesis, such as those of the polyomaviruses, must enter the nucleus despite continual transport of cellular proteins. Whether import of viral proteins is favored in such circumstances, for example, by the presence of high-affinity nuclear localization signals, is not known. Many viral structural proteins that enter infected cell nuclei form multimeric capsid components. Import of structural units of virus particles can depend on prior assembly in the cytoplasm to form the nuclear localization signal (see above) or to ensure efficient import. Assembly of the heteromeric complex facilitates import of the minor structural proteins, even though each contains a nuclear localization signal. The increased density of these signals may allow more effective competition for essential components of the import pathway, or the nuclear localization signals may be more accessible in the complex. Despite such potential advantages as increased efficiency of import of viral proteins and transport of the structural proteins in the appropriate stoichiometry, import of preassembled capsid components is not universal. However, when these two proteins are made in the absence of other adenoviral gene products, hexons do not enter the nucleus. Many viruses that are important human pathogens, including hepatitis B and C viruses, human immunodeficiency virus type 1, and influenza A virus, are enveloped. In fact, the particles of 50% of the virus families that reproduce in animal cells include a lipid membrane, regardless of the nature of the viral genome. Furthermore, acquisition of the envelope and release of these viruses from the host cell are frequently accomplished in a single step. In contrast, the particles of only some 10% of plant virus families are enveloped (3 of 29 listed in the Ninth Report of the International Committee on Taxonomy of Viruses [2012]). Two of these families, Bunyaviridae and Rhabdoviridae, also include viruses that replicate in animal cells, but with significant differences in assembly and release. In mammalian cells, rhabdoviruses, such as vesicular stomatitis virus, acquire their envelope, and are concomitantly released, by budding through the plasma membrane. However, plant rhabdoviruses form upon budding of internal components either into the endoplasmic reticulum (lettuce necrotic yellow virus) or through the inner nuclear membrane (potato yellow dwarf virus), and in both cases accumulate at these intracellular sites. In similar fashion, bunyavirus particles are released from infected animal cells via the secretory pathway following formation within Golgi compartments, but are not released from plant cells. For example, tomato spotted wilt virus particles accumulate in vesicles derived from Golgi and endoplasmic reticulum membranes until the cells are ingested by insect vectors (thrips) during feeding. In infected salivary gland cells of the insect host, tomato spotted wilt virus particles are formed and secreted from the plasma membrane like bunyavirus particles in mammalian cells. Formation of an envelope provides an effective means of direct or indirect release from animal cells of progeny virus particles, which can then infect other cells in the organism via their accessible plasma membranes. In contrast, plant cells are surrounded by a structure that imposes formidable barriers to exit and entry by these mechanisms, the cell wall. This thick and rigid structure is built from microfibrils of cellulose organized into a network with the polysaccharides pectin and cross-linking glycans (see the figure). Neighboring cell walls are penetrated by the numerous microchannels (plasmodesmata) by which a plant cell is connected to its neighbors. Consequently, the acquisition of an envelope is of little benefit to viruses that reproduce in plant cells. Rather, the genomes of all plant viruses encode movement proteins that induce alterations of plasmodesmata to allow direct passage of virus particles (or genomes) from one cell to another (Box 13. Furthermore, the great majority of plant viruses are transmitted among host plants not by release into the environment but by vectors, most commonly insects. Two adjacent plant cells showing the plasma membrane components of the cell wall and a plasmodesma through the plasma membrane and its internal tube-like structure, the dermotubule derived from the endoplasmic reticulum. Smooth endoplasmic reticulum Plasma membrane Cross-linked glycan Pectin Cellulose microfibrils Cell walls of adjacent plant cells Desmotubule Middle lamella Primary cell wall plant viruses (Box 12. Assembly of the majority of such enveloped viruses takes place at the plasma membrane. Before such virus particles can form, viral integral membrane proteins must be transported to this cellular membrane. The first stages of the pathway by which viral and cellular proteins are delivered to the plasma membrane were identified more than 35 years ago, and the process is now understood quite well.
Diseases
- Cerebellar hypoplasia
- Hereditary ataxia
- Treacher Collins syndrome
- Lipomucopolysaccharidosis
- Selenium poisoning
- Congenital nephrotic syndrome
- Chromosome 7, monosomy 7q21
Purchase norvasc 5 mg with amex
Additional internal (blue) density represents unidentified encapsidated proteins and/or misaligned capsid hypertension 7101 generic norvasc 10mg overnight delivery. As in the first template exchange, a particular organization of the template in the core particles is thought to facilitate the process. The first template exchange (step 2) is promoted by interaction with a sequence that lies between dr1 and dr2 at the 3 end of the pregenome. Mutation of the normal acceptor sequence leads to the synthesis of strands with 5 ends that map to other sites in the vicinity of the 3 dr1, which apparently can serve as alternative acceptors. A deletion analysis with the woodchuck virus has suggested that a region 1 kb upstream of the 3 dr1 includes a signal that specifies the acceptor site. At a low frequency (5 to 10%), the strand primer is extended in situ instead of being translocated (the structure set off by a dashed arrow) (step 5a); elongation of this strand results in a duplex linear genome. However, the mechanisms by which their templates are reverse transcribed are quite distinct. Differences in the form and function of the final products of the two pathways are especially striking. Repair of the circular intermediate is an unusual reaction, and covalently closed circle forms are dead-end products. The single-cell reproduction cycles of retroviruses and hepadnaviruses are, in a sense, permutations of one another. Retroid viruses appear to represent a continuum in evolution, and remind us of the varied combinations of strategies that exist in nature for replicating viral genomes and related genetic elements. It is now clear that these protein molecules can be scaffolds as well as catalysts, and their multiple functions appear to be enabled by a remarkable capacity for dynamic conformational change. It has been suggested that the simultaneous interaction of the central region with both ends may hold the termini in a position that facilitates both strand primer translocation and the second template exchange. The shaded boxes indicate that the nucleic acids (genomes) encapsidated in particles of each virus represent different components in analogous pathways. As might be expected, such progress has elicited important new questions to be addressed in the future. Human immunodeficiency virus reverse transcriptase: 25 years of research, drug discovery, and promise. The signals that control expression of the genes of these viruses are similar to those of cellular genes. Such orderly gene expression is primarily the result of transcriptional regulation by viral proteins. As a collateral dividend, we have gained insights into the cellular mechanisms that control progression through the cell cycle. Such conservation of sequence can be attributed to the common biochemical capabilities of the enzymes. Transitions from one phase to the next depend on viral activators and synthesis of progeny viral genomes. Viral proteins that regulate transcription may bind directly to viral promoter sequences or indirectly in association with cellular proteins. Some viruses, including the herpesviruses, establish latent infections in which transcription of lytic genes is inhibited and, in some cases, unique latencyassociated transcription units are expressed. Suppression of cellular transcription by viral components diverts limited cellular resources to aid viral transcription. Viral proteins can stimulate transcription of their own transcriptional unit to establish a positive autoregulatory loop or activate transcription of different viral genes. However, it can also carry out at least one reaction unique to virus-infected cells, Table 8. Transcription of specific genes is therefore the first biosynthetic reaction in cells infected by adenoviruses, herpesviruses, papillomaviruses, and polyomaviruses. The posttranslational modifications of the histones help distinguish highly condensed, transcriptionally silent heterochromatin from transcriptionally active genes. Application of this assay to herpes simplex virus type 1-infected cells, as illustrated, has demonstrated that histone H3 binds to immediate-early, early, and late genes in entering, but not in newly replicated, viral genomes. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. The polycomb group protein Bmi1 binds to the herpes simplex virus 1 latent genome and maintains repressive histone marks during latency. Temporal association of the herpes simplex virus genome with histone proteins during a lytic infection. Such nucleosomal organization suggests that mechanisms analogous to those regulating transcription of cellular chromatin are likely to operate on these viral templates. Indeed, as we shall see, the properties of viral "chromatin" can result in transcriptional silencing and prevent transcription of the majority of viral genes in cells latently infected by some herpesviruses. For example, the human adenovirus type 2 major late promoter was the first from which accurate initiation of transcription was reconstituted in vitro (Box 8. The core promoter comprises the minimal sequence necessary to specify accurate initiation of transcription. The activity of the core promoter is modulated by local regulatory sequences typically found within a few hundred base pairs of the initiation site. Distant regulatory sequences that stimulate (enhancers) or repress (silencers) transcription are present in a large number of transcriptional control regions. Application of these methods has identified a very large number of transcriptional control sequences. Core promoters of viral and cellular genes contain all the information necessary for recognition of the site of initiation and assembly of precisely organized preinitiation complexes. Such assemblies, termed holoenzymes, appear to be poised to initiate transcription as soon as they are recruited to a promoter. Many others must be maintained in an almost silent state, from which they can be activated rapidly in response to specific stimuli, and to which they can be returned readily. Transcription of viral genes is also regulated during the infectious cycles of most of the viruses considered in this chapter. Large quantities of viral proteins for assembly of progeny virions must be made within a finite (and often short) infectious cycle. In many cases, viral genes are transcribed in a specific and stereotyped temporal sequence. Indeed, it was detailed information about a particular adenoviral transcription unit that finally allowed biochemical studies of the mechanism of initiation. This knowledge was exploited to develop a simple assay for accurate initiation of transcription, the "runoff" assay, using a linear template that includes a transcription initiation site shown in the figure. This runoff transcription assay is convenient and has been used to assess both specificity and efficiency of transcription. In general, however, viral proteins are critical components of the circuits that establish orderly transcription of viral genes. Recognition of Local and Distant Regulatory Sequences Both local and distant sequences can control transcription from core promoters. However, local sequences are often sufficient for proper transcriptional regulation. An enormous number of sequence-specific proteins that regulate transcription are now known, many first identified through analyses of viral promoters. Unfortunately, the nomenclature applied to these regulatory proteins presents serious difficulties for both writer and reader, for it is unsystematic and idiosyncratic (Box 8. The first such enhancer, so named because it stimulated transcription to a large degree, was discovered in the genome of simian virus 40. Enhancers are defined by their position- and orientation-independent stimulation of transcription of homologous and heterologous genes over distances as great as 10,000 bp in the genome. Despite these unusual properties, enhancers are built with binding sites for the proteins that recognize local promoter sequences. The simian virus 40 enhancer has been studied intensively, and its properties and mechanism of action are characteristic of many enhancers, whether of viral or cellular origin. The severing of these contacts allows the transcribing complex to escape from the promoter and proceed with elongation (step 4). This promoter clearance step is often inefficient, with abortive initiation (step 5) predominating. In the latter process, initial transcripts are released, reforming the open initiation complex. The initial elongating transcriptional complex contains some but not all of the proteins that form the preinitiation complex, as well as proteins that stimulate elongation (not shown).
Discount 5mg norvasc with mastercard
This segment separates a larger external domain hypertension questionnaire questions purchase norvasc 5 mg overnight delivery, which is decorated with N-linked oligosaccharides (purple) and contains disulfide bonds (green), from a smaller internal domain. The high-resolution viral glycoprotein structures mentioned above are those of the large external domains of the proteins that had been cleaved from the viral envelope by proteases. This treatment facilitated crystallization but, of course, precluded analysis of membrane-spanning or internal segments of the proteins, both of which may operate structurally or functionally. Improvements in resolution achieved by application of cryo-electron microscopy or tomography have allowed visualization of these segments of glycoproteins of some enveloped viruses. These extracellular domains are depicted as they are oriented with respect to the membrane of the viral envelope. This small (97-amino-acid) protein is a minor component of virus particles, estimated to be present at 14 to 68 copies per particle. In the viral membrane, two disulfide-linked M2 dimers associate to form a noncovalent tetramer that functions as an ion channel. The effects of this drug, as well as of mutations in the M2 coding sequence, indicate that M2 plays an important role during entry by controlling the pH of the virion interior. Furthermore, the external domains of flaviviral E proteins are also icosahedrally ordered, and the envelopes of viruses of these families are described as structured. In contrast, as described in the next section, the arrangement of membrane proteins generally exhibits little relationship to the structure of the capsid when virus particles contain additional protein layers. Enveloped Viruses with an Additional Protein Layer Enveloped viruses of several families contain an additional protein layer that mediates interactions of the genome-containing structure with the viral envelope. Because the internal capsids or nucleocapsids of these more complex enveloped viruses are not in direct contact with the envelope, the organization and symmetry of internal structures are not necessarily evident from the external appearance of the surface glycoprotein layer. For example, the outer surface of all retroviruses appears roughly spherical with an array of projecting knobs or spikes, regardless of whether the internal core is spherical, cylindrical, or cone shaped. Likewise, influenza virus particles, which contain helical nucleocapsids, are generally roughly spherical particles but are highly pleomorphic with long, filamentous forms common in clinical isolates (Box 4. Although the interior architecture of these enveloped viruses cannot be described in detail, high-resolution structures have been obtained for several matrix proteins. In conjunction with the results of in vitro assays for lipid binding and mutational analyses, such information allows molecular modeling of matrix protein-envelope interactions. Internal proteins that mediate contact with the viral envelope are not embedded within the lipid bilayer but rather bind to its inner face. Such viral proteins are targeted to , and interact with, membranes by means of specific signals, which are described in more detail in Chapter 12. This inner protein layer is a T 4 icosahedral shell built from 240 copies of a single capsid (C) protein arranged as hexamers and pentamers. The outer glycoprotein layer also contains 240 copies of the envelope proteins E1 and E2, which form heterodimers. They cover the surface of the particle, such that the lipid membrane is not exposed on the exterior. The organization of the alphavirus envelope, including the transmembrane anchoring of the outer glycoprotein layer to structural units of the nucleocapsid, can therefore be described with unprecedented precision. This interaction accounts for the 1:1 symmetry match between the internal capsid and exterior glycoproteins. The boundaries of the structural (asymmetric) unit are demarcated by the red triangle, on which the icosahedral five-, three-, and twofold axes of rotational symmetry are indicated. This outer surface is organized as a T 4 icosahedral shell studded with 80 spikes, each built from three copies of each of the transmembrane glycoproteins E1 and E2. The lipid bilayer of the viral envelope is clearly defined at this resolution, as are the transmembrane domains of the glycoproteins. The C protein penetrates the inner leaflet of the lipid membrane, where it interacts with the cytoplasmic domain of the E2 glycoprotein (blue). The membrane is spanned by rod-like structures that are connected to the skirt by short stems. The view shown is around a quasithreefold symmetry axis, with the three E2 glycoprotein molecules in a trimeric spike colored light blue, dark blue, and brown and the E1 molecules shown as backbone traces colored red, green, and magenta. The portions of the proteins that cross the lipid bilayer are helical, twisting around one another in a left-handed coiled coil. Conserved domains of E and E1 are colored red, yellow, and blue with the fusion loops required for entry in orange. The parallel and perpendicular orientations to the membrane of the flavivirus and alphavirus envelope proteins, respectively, result in the very different appearances of these particles shown in panel B. The dimers related by two-, three-, and fivefold axes of icosahedral symmetry are colored blue, pale yellow, and mauve, respectively, except for the central dimer depicted, which is colored as in panel A. In the 80 spikes of the alphavirus envelope, E2 is shown gray and E1 colored by domain as in panel A. Such particles comprise obviously distinct components with different symmetries and/or multiple layers. In this section, we illustrate various ways in which multiple structural elements can be combined, using as examples bacteriophage T4, herpes simplex virus type 1, the poxvirus vaccinia virus, and Bacteriophage T4 Bacteriophage T4, which has been studied for more than 50 years, is the classic example of an architecturally elaborate virus that contains parts that exhibit both icosahedral and helical symmetry. The head of the mature T4 particle, an elongated icosahedron, is built from hexamers of a single viral protein (gp23*). In contrast to the other capsids considered so far, two T numbers are needed to describe the organization of gp23* in the two end structures (T 13) and in the elongated midsection (T 20). However, it is important to appreciate that the architectures reported are those of particles isolated and examined under a single set of specific conditions. Recent studies of the flavivirus dengue virus, an important human pathogen, illustrate the conformational plasticity of some mature virus particles. As noted previously, the E protein dimers are tightly packed and icosahedrally ordered. Indeed, when particles are exposed to temperatures encountered in the mammalian host. In fact, particles exposed to higher temperatures are heterogeneous, and the example shown in the figure (right) represents but one of multiple forms, identified during selection of particles for three-dimensional reconstruction. Because a heterogeneous population of particles with less well-ordered E protein dimers represent the form of dengue virus recognized by the human immune system, these observations have important implications for the design of dengue virus vaccines. The E protein dimers that lie at the twofold axes are shown in gray and the other dimers with one subunit in green and one in cyan. The particles exposed to higher temperatures are characterized by exposed patches of membrane (purple) and significant reduction of dimer contacts at the threefold axes of icosahedral symmetry. The outer layer is a contractile sheath that functions in injection of the viral genome into host cells. The tail is connected to the head via a hexameric ring and at its other end to a complex, dome-shaped structure termed the baseplate, where it carries the cell-puncturing spike. The former, which are bent, are the primary receptor-binding structures of bacteriophage T4. Herpesviruses Members of the Herpesviridae exhibit a number of unusual architectural features. More than half of the 80 genes of herpes simplex virus type 1 encode proteins found in the large (200-nm-diameter) virus particles. These proteins are components of the envelope from which glycoprotein spikes project or of two distinct internal structures. Influenza A virus particles exhibit even more extreme pleomorphism: they appear spherical, elliptical, or filamentous, and all forms come in a wide range of sizes (see the figure). Laboratory isolates are primarily filamentous but adopt the other morphologies when adapted to propagation in the laboratory. Several lines of evidence indicate that the filamentous phenotype is genetically determined. For example, the particles of some influenza A virus isolates are primarily filamentous, whereas those of other isolates are not. However, what determines the choice between assembly of filamentous versus spherical particles is not understood. Nor is the physiological significance of the filamentous particles, despite their predominance in clinical isolates. It has been speculated that these forms might facilitate cell-to-cell transmission of virus particles through the respiratory mucosa of infected hosts. The asymmetry of the herpesviral capsid and the incorporation of the portal have important implications for the mechanism of assembly (see Chapter 13).
Buy 5mg norvasc
One of the greatest current challenges in this field remains the elucidation of the mechanics of the movement of proteins arteria lingual generic norvasc 10 mg online, nucleic acids, nucleoproteins, or transport vesicles from one cellular compartment or site to another. The development and application of techniques that exploit fluorescent proteins to visualize transport in living cells is providing important new insights into these processes. The interaction probably takes place at the juxtanuclear sites where newly synthesized Gag accumulates. However, whether transport is direct, or via association with membrane-bound structures such as multivesicular bodies, or whether these different mechanisms dominate in different cell types is not yet clear. Indeed, nucleocapsids were observed to be distributed throughout the cytoplasm in close association with microtubules (panel A of the figure). Treatment of infected cells with drugs that disrupt microtubules, such as nocodazole, dramatically altered this pattern: nucleocapsids became clustered in the absence of microtubules in large aggregates and did not reach the plasma membrane (panel B). Such drugs also reduced virus yield significantly, confirming the importance of microtubules in the transport of vesicular stomatitis virus nucleocapsids to sites of assembly. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. A B microenvironments containing high concentrations of viral structural proteins and the nucleic acid genome. Such microenvironments are ideal niches for the assembly of progeny particles from their multiple parts. Flagging and docking: dual roles for N-glycans in protein quality control and cellular proteostasis. Making the case: married versus separate models of alphaherpes virus anterograde transport in axons. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Cooperation of structural proteins during late events in the life cycle of polyomavirus. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Kinesin-3 mediates axonal sorting and directional transport of alphaherpesvirus particles in neurons. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. Interactions of normal and mutant vesicular stomatitis virus matrix proteins with the plasma membrane and nucleocapsids. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Identification of a membranebinding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. Transport of Viral Proteins to Intracellular Membranes Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. Characterization of an endoplasmic reticulum retention signal in the rubella virus E1 glycoprotein. The membrane glycoprotein G1 of Uukuniemi virus contains a signal for localization to the Golgi complex. Nevertheless, successful reproduction of all viruses requires execution of a common set of de novo assembly reactions. In many cases, formation of internal virion structures must be coordinated with acquisition of a cellular membrane into which viral proteins have been inserted, or additional maturation steps must be completed to produce infectious particles. Assembly of even the simplest viruses is therefore a remarkable process that requires considerable specificity in, and coordination among, each of multiple reactions. In the extreme case of giant viruses, such as mimivirus and Pandoravirus, hundreds of proteins must interact appropriately with one another, with host cell membranes, and with the viral genome. Furthermore, virus reproduction is successful only if each of the assembly reactions proceeds with reasonable efficiency and if the overall pathway is irreversible. The diverse mechanisms by which viruses assemble represent powerful solutions to these problems associated with de novo assembly. Indeed, infectious virus particles are produced in prodigious numbers with great specificity and efficiency. Despite variations in structure and biological properties, all virus particles must be well suited for protection of the nucleic acid genome in extracellular environments. They must also be metastable structures, that is, built in a way that allows their ready disassembly during entry into a new host cell. A number of elegant mechanisms resolve the apparently paradoxical requirements for very stable associations among virion components during assembly and transmission but the ready reversal of these interactions when appropriate signals are encountered upon infection of a host cell. Like synthesis of viral nucleic acids and proteins, assembly of virus particles depends on host cell components, such as the cellular proteins that catalyze or assist the folding of individual protein molecules. Furthermore, the building blocks of virus particles are transported to the appropriate assembly site by cellular pathways (Chapter 12). Concentration of components of virus particles to a specific intracellular compartment or region undoubtedly facilitates virus production by increasing the rates of intermolecular assembly reactions. It is also likely to restrict the number of interactions in which particular components can engage, thereby increasing the specificities of these reactions. The survival and propagation of a virus in a host population generally require dissemination of the virus beyond the cells initially infected. Progeny virus particles must therefore escape from the infected cell for transmission to new cells within the same host or to new hosts. The majority of viruses leave an infected cell by one of two general mechanisms: they are released into the external environment in various ways, or they are transferred directly from one cell to another. Acquisition of an envelope by budding from the plasma membrane or internal membranes may be coordinated with, or follow, assembly of internal structures. Viral protein L domain sequences promote budding of enveloped viruses by recruitment of cellular proteins that participate in vesicular trafficking. When viral particles are assembled from polyproteins or precursor proteins, proteolytic cleavage by viral proteases is essential to produce infectious viral particles. Viruses may be released as free particles or spread from cell to cell without exposure to the extracellular milieu. Some structural units are formed from individual protein subunits; an alternative mechanism is assembly while individual protein coding sequences are covalently linked in a polyprotein precursor. An assembly line mechanism is well-suited for orderly formation of some virus particles. Accurate assembly of some large icosahedral protein shells requires scaffolding or chaperone proteins. Structural proteins contain the information necessary to specify assembly, but this process may be improved by the participation of cellular or viral chaperones. Assembly of the simian virus 40 capsid therefore must require specific variations in the ways in which pentamers associate, depending on their position in the capsid shell. Such subtle, yet sophisticated, regulation of the association of structural units was not anticipated and could be revealed only by high-resolution structural information. Reactions common to all viruses are shown in yellow, and those common to many viruses are shown in blue. The structural units that are often the first assembly intermediates are the homo- or hetero-oligomers of viral structural proteins from which virus particles are built (see Table 4. Packaging of the genome can be coordinated with assembly of the capsid or nucleocapsid, and for enveloped viruses, the assembly of internal components can be coordinated with acquisition of the envelope. Methods of Studying Virus Assembly and Egress Mechanisms of virus assembly and release can be understood only with the integration of information obtained by structural, biochemical, genetic, and imaging approaches. Structural Studies of Virus Particles the mechanisms by which virus particles form within, and leave, their host cells are intimately related to their structural properties. Our understanding of these processes therefore improves dramatically whenever the structure of a virus particle is determined. An atomic-level description of the contacts among the structural units that maintain the integrity of the particle identifies the interactions that mediate assembly and the ways in which these interactions must be regulated. For example, the X-ray crystal structure of the polyomavirus simian virus 40 described in Chapter 4 solved the enigma of Visualization of Assembly and Exit by Microscopy While high-resolution structural studies of purified virus particles or individual proteins provide a molecular foundation for describing virus assembly, they offer no clues about how assembly (or exit) actually proceeds in an infected cell.
Purchase norvasc 5 mg visa
Patients with type A disease usually die within a few years of birth; however blood pressure medication for ptsd buy generic norvasc 10mg on-line, patients with type B disease often live to adulthood and show progressive pulmonary infiltration, causing significant morbidity and often leading to death. Gaucher disease is characterized by lysosomal -glucosidase deficiency and the accumulation of glucocerebroside. Infantile, juvenile, and adult forms occur, with the adult form lacking neurologic involvement. Histologic Features Collections of foamy macrophages are a characteristic of lung 1351 involvement with storage diseases. They are located in the pleura, septa, airspaces, and interstitium, in comparison with predominantly airway-centered foamy macrophages seen in cases of obstruction and aspiration. On electron microscopy, the macrophages contain cytoplasmic giant lamellar structures. Gaucher Disease Foamy macrophages have eccentric nuclei and contain lipid forming a "wrinkled tissue paper" or "striated small rod-like" pattern. Cholesteryl Ester Storage Disease Alveolar and interstitial macrophages contain cytoplasmic lipid deposits. There are concentric intimal deposits of foam cells, with extracellular lipid and reactive fibrosis, within pulmonary arteries. Pleuritis can resolve; however, continued inflammation can result in a fibrinous exudative process involving the visceral and/or parietal surfaces known as fibrinous pleuritis. Fibrinous pleuritis can become organized, which is characterized by the formation of granulationtype tissue, sometimes referred to as organized fibrinous pleuritis. With further development and/or resolution of the inflammatory process at this stage, fibrosis can occur resulting in a thickened and fibrotic pleura in the area affected, which is termed fibrous pleuritis. A variety of etiologies can lead to the development of fibrinous and fibrous pleuritis including inflammatory conditions involving or adjacent to the pleura (pneumonia, pericarditis, hepatitis, peritonitis, and pancreatitis), connective tissue disorders, drug reactions, and malignancy. Pleural plaques can form as a result of infections, surgery, trauma, apical caps, and asbestos exposure. Pleural plaques occurring from asbestos exposure are often bilateral and symmetrical involving the lower lung fields. Microscopically, fibrinous material with its accompanying inflammation is seen along the pleura in fibrinous pleuritis. The mesothelial layer is oriented along the pleural surface which may show reactive and/or hyperplastic change (mesothelial 1358 hyperplasia). When granulation tissue is present, the proliferating capillaries and fibroblasts present may bring sarcomatous or desmoplastic mesothelioma into diagnostic consideration. Fibrinous and fibrous pleuritis is generally limited to and oriented along pleural surfaces; the malignant cells of mesothelioma will involve the entire surface and invade into adjacent tissues. Keratin immunohistochemical stains can be helpful to identify the location and orientation of the mesothelial cells in both conditions. In addition, the blood vessels seen with granulation tissue tend to be parallel to each other and perpendicular to the pleural surface. Fibrous pleuritis is characterized by a fibrous pleura; occasional scattered inflammatory cells are often seen. Pleural plaques consist of virtually acellular collagen bundles, often in a characteristic basket weave pattern. Histologic Features Fibrinous pleuritis consists of fibrinous material with accompanying inflammatory cells. The mesothelium can be reactive; accompanying mesothelial hyperplasia can be seen. The mesothelial cells should be oriented along the pleural surface (can be highlighted with immunohistochemical stains for keratin markers). Blood vessels of granulation tissue are parallel to each other and perpendicular to the pleural surface. As fibrous tissue becomes well developed a plaque may form, characterized by virtually acellular collagen bundles in a basket weave pattern. The small blood vessels that are present are parallel to one another and perpendicular to the surface of the pleura. Cagle Similar to the descriptive terminology used with fibrinous and fibrous pleuritis, descriptive terms have been used to characterize other forms of pleuritis with particular microscopic findings. Eosinophilic pleuritis is characterized by numerous eosinophils in the pleural fluid and/or tissue and tends to occur with pneumothorax or hemothorax. Given the association, it is not surprising that eosinophilic pleuritis is also often seen following pleurectomy or surgery to excise blebs and bullae. The cause of eosinophilic pleuritis is not limited to air and/or blood exposure as it has been described with certain infections (parasite, tuberculosis, fungus, and some bacteria), drug reactions, and can be seen in women of child-bearing age having lung tissue affected by lymphangioleiomyomatosis. Granulomatous pleuritis is characterized by granulomatous inflammation and/or well-defined granulomas with or without necrosis involving the pleura. Associations included certain infections (particularly fungus and tuberculosis), sarcoidosis, granulomatosis with polyangiitis (formerly known as Wegener granulomatosis), and secondary to foreign-body material. Foreignbody material is sometimes instilled into the pleural space in efforts to treat recurrent pleural effusions by obliterating the potential pleural space. Histologic Features Eosinophilic Pleuritis Eosinophils infiltrating pleural tissue; associated reactive mesothelium, histiocytes, lymphocytes, and giant cells are often seen. Adjacent lung tissue may have scattered eosinophils, particularly within small vessels. Underlying or adjacent lung may show bullae, honeycomb change, or lymphangioleiomyomatosis. Granulomatous Pleuritis Granulomatous inflammation and/or well-formed granulomas with or without necrosis involving the pleura. Special stains for fungal (for example, Gomori methenamine silver) or acid-fast (Kinyoun or auramine) organisms can help identify microorganisms; it is important to remember that a negative stain does not exclude the presence of organisms. Sometimes, foreign material may be identified, particularly with polarization; foreign material is sometimes used to obliterate the potential pleural space to prevent and treat recurrent pleural effusions. Fraire Eosinophilic pseudovasculitis is a subpleural infiltration of vascular walls by eosinophils. There is a described association with pneumothorax; however, the exact etiology is not known. One proposed theory is a defect in vascular transport of eosinophils migrating toward injured pleura. Typically, the eosinophilia seen is most pronounced in vessels close to the pleura and diminishes in those moving away from the pleural surface. Histologic Features Transmural infiltration of eosinophils in vessels near the pleura; diminishing quantity of eosinophils in vessels moving away from the pleura. Note also the absence of necrosis and a cluster of eosinophils within the vascular lumen. Cagle the mesothelium can become reactive and undergo hyperplasia in response to a variety of conditions including pleural effusion, infections, pneumothorax, surgery, trauma, connective tissue disease, and drug reactions. When present, reactive mesothelial cells can be abundant in pleural fluid cytology specimens showing significant cytologic atypia and even mitotic figures. Surgical biopsy specimens can also be quite challenging, particularly when mesothelial proliferations are entrapped by overlying fibrinous and/or fibrous pleuritis which can mimic invasion. Often, a multimodality approach is needed to correctly classify the process taking clinical, radiographic, and pathologic findings into account. Small biopsy and cytology specimens can be quite problematic sometimes requiring multiple different samples before definitive classification is made. Cytologic Features Mesothelial cells are typically cuboidal to round with variable cell and nuclear size/shape. Cytoplasm can be vacuolated to foamy or dense; the peripheral clear rim or "skirt" appearance (resulting from long microvilli processes visible by electron microscopy) helps to appropriately classify the cells.