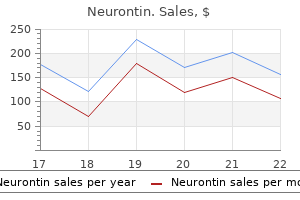
Best buy for neurontin
In addition symptoms concussion purchase neurontin once a day, it is important to note that the diabetes duration of patients in this study was relatively short (3. Most of these studies are cross-sectional in nature but more recently prospective studies proving causality have been published. It is likely that both recurrent hypoxia [95] and recurrent arousals [96] contribute to the activation of the sympathetic system. In another important prospective study, 1022 patients were followed up for a median of 3. Measures of intermittent hypoxemia, but not sleep fragmentation, were independently associated with all-cause mortality [156]. They work by pulling the tongue forward or by moving the mandible and soft palate anteriorly, enlarging the posterior airspace, which results in opening and increasing the airway size. If the patient has upper airway obstruction (such as tonsils or tumors) then surgery is the most important aspect of the treatment, otherwise its role is limited and it is usually associated with significant side effects [160]. In a study of 3565 participants, who were followed up for a 6-year period, the presence of a history of witnessed apneas was an independent predictor of incident sleep apnea [162]. This suggests that not only can sleep apnea result in dysglycemia as shown by longitudinal studies, but that pre-existing dysglycemia also predicts the development of sleep apnea. However, prospective and interventional studies are required to confirm causality. Lavie L: Oxidative stress-a unifying paradigm in obstructive sleep apnea and comorbidities. Wang X, Bi Y, Zhang Q, Pan F: Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Young T, Blustein J, Finn L, Palta M: Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Terбn-Santos J, Jimenez-Gomez A, Cordero-Guevara J: the association between sleep apnea and the risk of traffic accidents. Broadly, insulin resistance can be defined as an abnormal biologic response to insulin; insulin, whether endogenous or exogenous in origin, has limited ability to reverse a hyperglycemic metabolic state. Due to the very close link between diabetes and insulin resistance, no formal clinical definition of insulin resistance has emerged. We will discuss the potential clinical use of the insulin resistance syndrome and the metabolic syndrome later in the chapter. For now, we focus on investigation of insulin resistance as a useful entity for research concepts, and in particular discuss its biologic consequences by describing its associated risk factor perturbations. With increasing adiposity, fat cells become enlarged and less responsive to insulin, that is, they become insulin resistant. Excess fat in the latter two organs is considered to enhance their insulin resistance; International Textbook of Diabetes Mellitus, Fourth Edition. The figure illustrates the relevance of insulin action not simply to skeletal muscle glucose uptake, but also adipose, liver, endothelium, and immune cell function. As fat cells enlarge with greater obesity and become more insulin resistant, they enhance. Similarly, a subclinical "fatty liver," dysfunctional endothelium, and irritated immune cells in insulin resistance release an array of molecules into the circulation. As a result, the circulating concentrations of such parameters give indications as to the degree of insulin resistance and also therefore associate with future risk for type 2 diabetes. This hyperglycemia can be offset for many years by an increase in pancreatic insulin secretion, and hence hyperinsulinemia accompanies insulin resistance. The major drawback of this gold-standard approach is its labor-intensive and time-consuming nature. Thus, it is not a practical test for routine screening or for large epidemiologic studies. Other dynamic tests to measure sensitivity to insulin are available but are infrequently used in clinical settings (Table 23. Of all of these, rising obesity levels are strongly associated with insulin resistance whatever the other characteristics of an individual and, as such, fat accumulation is the primary risk factor that drives increasing insulin resistance at a population level. In other words, nonmodifiable characteristics (age, sex, ethnicity, family history) interact with adiposity to determine degree of insulin resistance and How do we measure insulin resistance? The gold standard method for measuring insulin resistance focuses on maintaining a normal blood glucose level in a hyperinsulinemic state-the so-called euglycemic hyperinsulinemic clamp. This technique involves infusing insulin at supraphysiologic rates based on body size, while also infusing glucose at the necessary rate to maintain euglycemia. The use of stable isotopes provides further information on the relevant roles of the liver and peripheral tissue (muscle and adipose tissue). When a steady state is achieved, the glucose infusion rate is essentially a measure of the ability of all tissues to respond to insulin and take up glucose for a standard insulin dose [1]. Thus, the global sensitivity to insulin is comparable between patients or participants. There are several different specific the insulin resistance syndrome 341 Table 23. Radio-labeled glucose tracer can be used to investigate tissue-specific glucose disposal. Glucose is infused (with or without insulin) and glucose is measured on multiple occasions over 3 h. Blood samples for glucose and insulin measures are drawn at a few time points after around 1 h. This is a routine test for the clinical diagnosis of diabetes when simpler blood tests are ambiguous. Often involves a 75 g or 100 g dose of glucose, and measuring glucose and insulin at frequent intervals. Since fasting insulin levels are skewed, log-normalized data can be used in models. Pathways linked to insulin resistance From the preceding observations on the actions of insulin and the role of ectopic fat in exacerbating insulin resistance, a number of linked metabolic perturbances can be predicted. The most clinically relevant and interesting pathways are discussed later with an attempt to draw out potential causal associations where relevant. Whilst this lipid pattern is often considered to be linked to increased vascular risk in the prediabetes individual, the causal nature of these associations remains debated. Furthermore, it is now clear that elevated triglyceride levels in most individuals more strongly indicate diabetes than vascular risk [7]. Despite this, evidence from a large genetic study is potentially consistent with a causal role for triglycerides in heart disease [10]. Thus it is possible for a risk factor to be causal, but offer redundant information in terms of risk scores. Interestingly this characteristic, in turn, may be a downstream consequence of "impaired" subcutaneous fat storage capacity, the mechanisms of which deserve further research. As an extreme example of this concept, lipodystrophic individuals have an impaired ability to store subcutaneous fat and, as a consequence, they accumulate fat in visceral and ectopic tissues and so have marked insulin resistance [3]. It is also apparent that certain ethnic groups at heightened diabetes risk may have a tendency to store fat centrally sooner. Imaging studies have shown these individuals to have low levels of visceral and ectopic fat but a high subcutaneous fat content [5]. The insulin resistance syndrome 343 Excess caloric intake Subcutaneous Retroperitoneal 1. This figure provides a simple conceptual illustration of the development and location of ectopic fat (and thus worsening insulin resistance) in individuals once they have "overwhelmed" their ability to store safe subcutaneous fat. Certain factors such as gender (females having greater storage capacity), genetics (with family history of diabetes as a broad proxy measure), ethnicity. In temporal terms, it may be liver fat accumulation is closer to the time of development of diabetes whereas muscle insulin resistance is an earlier development. Perivascular fat may contribute to vascular dysfunction via a process of adverse vasocrine signaling leading in turn to impaired nutrient blood flow-that is, vascular insulin resistance. Finally, some recent evidence indicates excess fat may also accumulate in the pancreas to contribute to -cell dysfunction, and thus development of diabetes. Several factors released by the liver have been associated with insulin resistance as shown in Table 23. Further basic and clinical work in this area would be useful to tease apart the causal pathway. Recent evidence indicates that Fetuin-A may be implicated in insulin sensitivity through inflammatory signaling pathways [20].
Cheap 400mg neurontin fast delivery
The precipitating factors include dehydration symptoms multiple myeloma order 100mg neurontin mastercard, fever, stress, acidosis and exposure to cold. Few patients require hospital admission and intravenous morphine for pain control. Blood transfusion is generally not required except if there is a decrease in hemoglobin, respiratory distress or hypotension. Parents should to sensitized to assess the splenic size and obtain prompt medical care in case of a rapid splenic enlargement. In India, lot of children with sickle cell disease have large spleens which do not undergo involution. Management includes oxygen therapy and rapid correction of anemia with blood transfusions. The parvo virus B19 infection can present with slowly developing anemia over 2-4 weeks with or without leukopenia and thrombocytopenia. The management depends on the severity of anemia and the clinical status of the patient. Packed red blood cells, if required, should be transfused slowly over a period of 4 hours in small aliquots. In patients not responding to these measures, aspiration of blood from corpora cavernosa and irrigation with epinephrine is recommended. It is also recommended for patients who continue to experience recurrent severe acute complications. The potential adverse effects of chronic transfusion therapy are alloimmunization, iron overload and transfusion transmitted infections. The thalassemia refers to decreased or absent production of chains and thalassemia results from decrease or absence of globin chain. Thalassemia thalassemia is an autosomal recessive condition that varies in severity from thalassemia trait to a thalassemia major where a patient requires lifelong transfusion. Pathophysiology chains are either reduced or not produced in a patient with thalassemia major. Ineffective erythropoiesis gives rise to hepatosplenomegaly and expansion of flat bones resulting in characteristic chipmunk facies. Along with iron from the blood transfusions, this results in iron overload in the body producing damage to heart, liver and endocrine organs like pancreas, thyroid and pituitary. Older children can present with facial dysmorphism and complications of thalassemia like growth failure, short stature, endocrine disorders. Laboratory Investigations Diagnosis is based on hematological features coupled with hemoglobin analysis and molecular studies. A complete blood picture shows microcytic, hypochromic anemia with presence of anisocytosis, poikilocytosis, target cells and erythroblasts in a peripheral blood film. The findings of hemoglobin analysis in different forms of thalassemia are described in Table 5. Reverse dot blot or restriction enzyme analysis can identify one of the common mutations in thalassemia. Management the appropriate management of a patient with thalassemia requires a multidisciplinary team consisting of a pediatrician, hematologist, gastroenterologist, cardiologist, endocrinologist, gynecologist and a hemoglobinopathies and hemoglobin Disorders psychologist. The role of a pediatrician is pivotal in diagnosis, blood transfusion, iron chelation therapy, monitoring and timely recognition of complications the management has four important components-blood transfusion, iron chelation, monitoring for complications and prevention of further births of thalassemia homozygotes in the family. Most centers follow moderate transfusion regimen as it leads to appropriate growth and development with minimal iron overloading. Leucoreduced packed red blood cells ideally cross-matched for minor antigens including at least C, c, D, E, e, and Kell. This coupled with increased gastrointestinal absorption in patients with thalassemia result in iron accumulation in the body. Iron Chelators Three iron chelators are available: desferrioxamine, deferiprone and deferasirox. It is first iron chelator to be introduced in 1960s and remains the gold standard iron chelator till date. The benefit of desferrioxamine is proven by the improved survival of successive cohorts of thalassemia major born after 1970. Special equipment required Once daily administration Cost hemoglobinopathies and hemoglobin Disorders shown to improve cardiac and hepatic iron overload gradually over a period of time. Combination results in greater reduction in body iron than either of the drugs alone. Larger studies are required to establish the efficacy and safety of these combinations. Serum ferritin Serum ferritin is a readily available, inexpensive, indirect measure of body iron stores. Serum ferritin has been shown to correlate with the body iron stores in thalassemia major but underestimate the iron loading in thalassemia intermedia. Serum ferritin can be falsely elevated in inflammation, hepatitis and depressed in vitamin C deficiency. Serum ferritin is measured serially every 44 hematology and Oncology 3 months in patients with thalassemia major and chelation therapy tailored accordingly. A trend in serum ferritin is more important than a single value while deciding upon the chelation regimen. Liver iron concentration Liver iron concentration is the reference standard for estimating the total body iron. Only 4 such machines are available worldwide, limited availability, high maintenance cost and requirement for dedicated trained staff limits its widespread utilization. It can also give additional information regarding the chamber size and function at the same time. Detection of Complications Growth and development: Growth failure is common in patients with thalassemia. Before the advent of iron chelation therapy, most patients would succumb to cardiac failure in the second decade of life. It is recommended to do the following investigations annually in patients older than 10 years. Chronic liver disease due to all these factors can result in cirrhosis and hepatocellular carcinoma. Liver biopsy can be done to assess degree of fibrosis, hepatitis activity and the iron overload. Fibroscan is a noninvasive technique to assess the liver fibrosis and can be used in thalassemia patients with chronic hepatitis C. Endocrinopathies Endocrinopathies are the most common complications of thalassemia. Short stature and delayed puberty can occur in spite of regular blood transfusion and iron chelation. The incidence shows various endocrinopathies in a cohort of 3817 thalassemia major patients across 29 countries in a study by De Sanctis et al. The investigations recommended for monitoring of various endocrinopathies are described in Table 5. Osteoporosis: Despite adequate blood transfusion and iron chelation, bone disease remains a major cause of morbidity and mortality in patients with thalassemia major. The frequency of low bone density increases with age, hypogonadism, iron overload and lack of physical activity. It is recommended to monitor serum calcium, phosphorus, parathyroid hormone, vitamin D every 46 hematology and Oncology Table 5. Role of Splenectomy Splenectomy is not routinely recommended for thalassemia patients as it is associated with long-term adverse effects like sepsis, thromboembolism and pulmonary hypertension. Role of Hematopoietic Stem Cell Transplant Hematopoietic stem cell transplant is the only available cure for thalassemia major as of today. Alternative donor transplants like matched unrelated donors and cord blood transplants are being done at many centers. The functional globin gene is introduced into the primitive hematopoietic stem cells using a lentivirus vector. These stem cells are then reintroduced into the host after a nonmyeloablative conditioning. Chapter 6 Aplastic Anemia Shirali Agarwal, Anupam Sachdeva Introduction Aplastic anemia is a physiologic and anatomic failure of the bone marrow, characterized by a marked decrease or absence of blood-forming elements in the marrow and pancytopenia.
Neurontin 100 mg generic
The resultant anemia is contributed to by both ineffective erythropoiesis and a component of hemolysis medicine ball core exercises order neurontin 100mg otc. However, occasional cases with severe neonatal anemia, neonatal jaundice and hydrops fetalis have been described. Neonatal anemia 233 Infection Intrauterine infections with many pathogens like cytomegalovirus, rubella, herpes simplex virus, toxoplasmosis and syphilis can cause neonatal anemia. However, hemolysis rather than impaired erythropoiesis is the predominant contributor to anemia in these instances. Intrauterine infection with human parvovirus B19 following maternal infection with the virus causes profound suppression of fetal and neonatal erythropoiesis with resultant anemia and can lead to non-immune fetal hydrops. Fetal loss is highest when the infection occurs in the initial 20 weeks of gestation. Hydrops fetalis can be diagnosed in these cases by fetal ultrasonography in the second trimester, and appropriate intervention, including intrauterine transfusions, can be performed to salvage the fetus. Nutritional Deficiencies Deficiencies of nutrients essential for erythropoiesis are quite rare in the fetus as it is an efficient parasite during intrauterine life. However, preterm neonates are more susceptible to these deficiencies due to decreased time availed to them to obtain these nutrients as well as the rapid catch-up growth they exhibit following delivery. The more frequently encountered nutritional deficiencies are briefly outlined below. Due to the previously mentioned factors, the preterm neonate is more vulnerable to this problem. Folate deficiency Preterm infants are commonly deficient in folate stores and can quickly develop anemia in the absence of adequate folate supplementation. Vitamin B12 deficiency Intrauterine absorption of vitamin B12 is the main source for stores in the neonate. Supplementation, by the intramuscular route if necessary, is advisable in these cases to avoid anemia as well as neurologic deficits. This is regarded as a physiologic adaptation following birth and is termed as the physiologic anemia of infancy. The speed of onset and degree of anemia is directly proportionate to the prematurity of the newborn. This analogous anemia in the preterm neonate is known as the anemia of prematurity. Diagnosis of Neonatal Anemia Neonatal anemia may present with one or more of the following features: 1. Coincidentally detected low Hb Accurate diagnosis of neonatal anemia with identification of primary etiology is important to ensure appropriate therapeutic measures are instituted. Hence, a detailed history, thorough physical examination and in most cases, a welldirected laboratory evaluation are important to arrive at the correct diagnosis. History A detailed maternal history for pre-existing medical conditions, medications and review of available previous maternal investigations is often helpful. Records of birth history including gestation at birth, method of delivery, complications (if any) and antenatal evaluations should be revisited. Current medical history of the affected neonate including age at presentation with anemia and other co-existing clinical features should also be assessed. Examination the affected neonate should be subjected to a proper physical examination to identify any specific findings related to the various disorders discussed above Neonatal anemia that may aid in diagnosis. These may include (but not be limited to) jaundice, organomegaly, lymphadenopathy, skin lesions, congenital malformations or other notable physical findings. Further investigations should be guided dependent on the information obtained from history, clinical examination and baseline test results. A diagnostic flow diagram to guide further assessment in neonatal anemia is provided below (Flowchart 24. Also, the clinical condition of the affected neonate is central to deciding immediate therapeutic interventions. The discussion below initially outlines general therapeutic measures and specific treatment based on particular etiologies is then briefly outlined. The goal of transfusion should be to maintain or restore oxygen delivery to tissue without causing cardiac compromise or increased oxygen consumption. In view of the inherent risks associated with transfusion of blood components (transfusion transmitted infections, transfusion reactions etc. Neonates can become iron-deficient due to increased demand, chronic blood loss (bleeding or hemolysis) or impaired storage (as in prematurity). However, indiscriminate iron supplementation should be avoided in view of increased risk of oxidantmediated damage in preterm neonates. Adequate supplementation of vitamin E and ascorbate may be protective against iron-mediated oxidative damage. Also, simultaneous supplementation of vitamin B12 and folic acid is advisable, especially in breastfed preterm neonates. The importance of minimizing phlebotomy losses in these neonates cannot be overemphasized. Consequently, management of hyperbilirubinemia in the neonatal period is of utmost importance. Lifelong supplementation of folic acid is advisable and a role for splenectomy in later life should be considered. Exposure to oxidant drugs and compounds, including maternal exposure to these compounds, should be avoided. Appropriate doses of vitamin K using the fat-soluble preparation (phytomenadione) should be provided to minimize the risk of bleeding. Hemoglobinopathies Initiation of penicillin prophylaxis, adequate disease education and appropriate referral to comprehensive disease-specific patient care program is 238 hematology and Oncology important in neonates diagnosed with sickle cell disease. The subcutaneous route of administration is used more frequently although the intravenous route has also been advised. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Various fetomaternal, neonatal conditions and mechanisms are recognized to have association with neonatal thrombocytopenia. Thrombocytopenia is attributed to impaired platelet production, consumption and sequestration or a combination of all these mechanisms. Less commonly, healthyappearing neonates who have thrombocytopenia may have a condition that is not expressed fully in the newborn period. Neonatal thrombocytopenia 241 Neonatal Immune Thrombocytopenia4 Immune thrombocytopenia is defined as an increased rate of platelet clearance caused by platelet-associated immunoglobulin G (IgG) or complement. It is the most common form of increased platelet destruction in newborns and should always be suspected in otherwise healthy infants with isolated severe thrombocytopenia. Neonatal immune thrombocytopenia can be further divided into three types depending on the characteristics of antibody. Transplacental passage of a maternal antibody directed against a non-self platelet antigen (termed "alloimmune thrombocytopenia" or sometimes "isoimmune thrombocytopenia"), transplacental passage of a cross-reactive maternal autoimmune-derived antiplatelet antibody, or generation of an autoreactive antiplatelet antibody by the newborn itself (termed "autoimmune thrombocytopenia"). The estimated incidence is about 1 in 1000 to 5000 livebirths in white population. It arises when the mother, who lacks a common platelet antigen, is exposed to the antigen (inherited from the father) on neonatal platelets and generates a neutralizing IgG. This crosses the placenta and mediates premature clearance of fetal and newborn platelets. Maternal or fetal serum also can be examined for the presence of antiplatelet antibody. This diagnosis should be considered when both the neonate and the mother have evidence of thrombocytopenia or in infants of mothers with a previous history of immune thrombocytopenia. Thrombocytopenia in Sick Neonates, Preterm Infants and who have Other Medical Complications5 Numerous conditions are associated with low platelets. For example, newborns who suffer hypoxia or acidosis after birth trauma often have low platelet counts, as do those who experience intrauterine growth retardation and chronic hypoxia from placental insufficiency. Viral infections in the perinatal period can cause severe thrombocytopenia as a result of intravascular platelet aggregation, and decreased production from degeneration of megakaryocytes. However, most of the episodes are mild or moderate and resolve spontaneously without any apparent sequelae. In secondary hypertension, often a well-defined cause is responsible for the raised blood pressure such as hyperaldosteronism or renal artery stenosis. However, in the vast majority of hypertensive patients, no obvious cause is found. In this chapter we will examine the different pathophysiological processes that are thought to cause the development and progression of high blood pressure.
Cheap 100 mg neurontin with visa
Chapter 6 provides a detailed description of the role of adiposity in glucose regulation medications janumet neurontin 100mg. Others have reported that weight reduction seems to be beneficial regardless of the mechanism of weight loss. Undertaking insufficient physical activity is associated with over 20 diseases and conditions and is estimated by the World Health Organization to be the fourth leading cause of premature mortality globally [38], ahead of both obesity and dietary factors. Observational research has confirmed that physical activity is a strong determinant of health in those with prediabetes. For example, in a large international cohort with prediabetes every 2000 steps per day change in walking activity over a 12-month period, as measured by a pedometer, was associated with an 8% difference in the risk of cardiovascular disease [39]. Others have shown that undertaking 150 minutes of leisure time walking activity per week was associated with a 60% reduction in the relative risk of diabetes [40]. Mechanistic studies have identified multiple pathways linking physical activity to insulin-stimulated glucose uptake [4143]. Furthermore, muscle is an active endocrine organ and releases a plethora of myokines which are regulated by muscle fiber activation. Physical activity acts to favorably alter the distribution and composition of adiposity within the body. For example, increased physical activity is associated with reduced levels of hepatic fat, even without whole body weight loss, and recent evidence points to the role of physical activity in the promotion of brown adipose tissue [45,46]. This is consistent with other studies and the results of a meta-analysis which demonstrated that physical activity interventions result in around a 50% reduction in the relative risk of type diabetes and are as effective as other multifactorial interventions that also promoted a healthy diet and weight loss [24]. As mentioned earlier, many of the benefits of increased physical activity are independent of overall body weight and adiposity. Observational research and interventions have consistently demonstrated substantial improvements in glucose regulation and reduced cardiovascular disease risk with higher levels of physical activity despite no significant change to body weight or waist circumference [39,40,42,47,49]. Therefore it is clear that the promotion of physical activity significantly improves glucose regulation, even in the absence of weight loss. Successful diabetes prevention programs have typically promoted at least 150 minutes of moderate intensity physical activity per week, which is consistent with current international and national physical activity recommendations for health [50,51]. Diet In addition to weight loss, diabetes prevention programs have targeted a reduction in total and saturated fat, with increased fiber intake. Plausible mechanisms linking these factors to insulin action have been proposed, including reduced ectopic fat, reduced low-grade inflammation, changes in cell membrane phospholipids, and enhanced intestinal peptide secretion [54,55]. As well as specific dietary factors, observational studies have suggested that overall dietary patterns are also an important consideration. In addition, we highlight some new classes of drugs that have potential in the prevention of diabetes. It is classed as a biguanide and acts through suppressing hepatic glucose production and inhibiting free fatty acid production and oxidation, therefore reducing insulin resistance and promoting peripheral glucose uptake [58]. Metformin is known to have some gastrointestinal side effects (diarrhea, flatulence, nausea, and vomiting), however the therapy is generally well tolerated and these side effects are typically not treatment limiting. As well as the primary effect on glucose uptake, several relevant pleiotropic effects have been postulated for metformin, specifically the prevention of cardiovascular disease and cancer [60,61]. However, whilst each of these potential benefits has supporting observational and mechanistic evidence, data from placebo-controlled randomized trials is lacking or inconclusive. In addition, rosiglitazone significantly increased regression to normoglycemia by 7080% compared to placebo. Other studies in those with prediabetes have been shown to have similar effects [69]. However, no published study has yet been specifically designed to assess this outcome. Meglitinides Nateglinide is a D-phenylalanine analogue, which acts directly on the cells to stimulate a rapid short-duration secretion of insulin, thereby controlling postprandial hyperglycemia. At 5 years, no significant difference was seen between placebo and nateglinide in these primary outcomes. In total, 12,537 patients with cardiovascular disease or at high risk for cardiovascular event who had prediabetes or diabetes were randomly assigned to receive insulin glargine, N-3 fatty acids supplementation or placebo. However, rates of weight gain and hypoglycemia were greater in the insulin glargine group and treatment was not Prevention of type 2 diabetes 557 associated with a reduction in cardiovascular events after 6 years in the study cohort overall [77]. However, low rate of completion (52% in the orlistat group) limits the conclusions that can be drawn from this study suggests poor acceptability among participants. For example, just 20 weeks of liraglutide therapy has been shown to be more effective than orlistat in the treatment of obesity and resulted in a reduced prevalence of prediabetes by 8496% depending on the dosage used [80]. Lifestyle versus pharmacotherapy Although national organizations and regulatory authorities are increasingly recommending the use of metformin, with the addition of other agents likely in the future, there remains some controversy around the use of pharmaceutical intervention as an adjunct, or in addition, to lifestyle intervention. First, few studies have assessed whether an additive benefit is gained by using lifestyle and pharmaceutical intervention in combination. Second, some drugs may actually act to attenuate the beneficial effects of lifestyle intervention. For example, there is mounting evidence that metformin may blunt the insulin sensitizing effects of exercise training in those with prediabetes or diabetes [82,83]. For example, physical activity is associated with a reduced risk of all-cause mortality, a reduced risk of cardiovascular morbidity and mortality, increased physical functioning and a reduced risk of depression [84]. Therefore, lifestyle modification programs should be the focus of diabetes prevention initiatives. Whilst logistical and feasibility issues remain in implementing lifestyle programs in a primary healthcare or community settings (see following sections), it is the responsibility of funding bodies and research organizations to address this need. However, we acknowledge that pharmacotherapy may have a role to play when lifestyle modification programs have been tried and found to fail. Cost-effectiveness Cost-effectiveness modeling studies using different methods and assumptions have consistently found, based on results from the major prevention trials, that lifestyle intervention and metformin are likely to be cost-effective or incur a modest cost saving [85]. Importantly, when considering the whole process from screening to treatment, Gillies et al. Further risk scores have been adapted to , and validated cross-sectionally in, diverse populations. However, recent innovations have included the development and validation of risk scores specially designed for use on data which is routinely available to and stored by general practitioners [9799]. Two-stepped approach Given their ease of use and noninvasive methodology, diabetes risk scores are now routinely used within many healthcare organizations. This has necessitated a parallel shift in the focus of diabetes prevention research, to include the translation of effective prevention strategies into routine clinical practice [88]. This work has highlighted several key considerations around two specific areas: (1) suitable strategies to identify high-risk individuals, and (2) the incorporation of lifestyle interventions into routine clinical services. In addition, there are clinical concerns around patient compliance to fasting procedures and the test-retest reliability of 2-hour glucose values. It is also known that the risk of cardiovascular disease increases linearly with increasing levels of 2-h glucose and fasting glucose [91] and there does not appear to be a distinct threshold that justifies the use of distinct risk categories. Despite the potential clinical utility of diabetes risk score technology and simple blood tests in the identification of high-risk populations for referral into diabetes prevention initiatives, it is important to recognize that such approaches are not yet supported by gold-standard evidence. However, there is huge gap between evidence-based effectiveness and what is feasible in a real-world healthcare setting. Large clinical trials are conducted in ideal settings and apply significant and concentrated resources in pursuit of their goals [88]. This degree of intervention intensity is far greater than even that offered to those with serious diseases in routine clinical care. Therefore, as others have noted, emphasis needs to be placed on examining the minimum level of intensity needed to produce meaningful clinical effects [101]. Beyond that, there are considerations around how new interventions become imbedded within usual care pathways and gain universal access. More recently, Schwarz and colleagues identified six key areas of focus to ensure sustainability when implementing diabetes prevention programs into practice, including: (1) intervention cost, (2) training and expertise of intervention providers, (3) uptake to both screening and intervention, (4) ensuring sustainability of funding and support within healthcare and political arenas, (5) developing quality management across intervention providers, and (6) using and improving technology to support behavior change of both patients and healthcare professionals [54]. Finland adopted a regional systematic whole system approach across all sectors of the healthcare community and included population-based strategies, high-risk strategies, and early diagnosis and management strategies [103]. This program, consisting of 16 1-hour group-based sessions delivered by trained quality assured lifestyle coaches, has gained public and private support and has been integrated into the healthcare package offered by some health-insurance companies. Internationally, European-wide diabetes prevention guidance and tools for healthcare professionals have also been developed and published [106]. Other groups have also recently begun to design and evaluate interventions that are specifically tailored to the structure and resources available to their national healthcare services. This theory-driven program, titled "Walking Away from Type 2 Diabetes," has been fully developed into a nationally available diabetes prevention program with accredited educator training and quality assurance professional pathways [107]. Intervention designed to translate the findings from diabetes prevention trials into practice, although developed concurrently across diverse healthcare settings and populations, have commonality across several key areas: they have concentrated on lifestyle, tended to utilize group-based education and counseling approaches, and have been delivered across a range of primary care and community venues by personnel largely outside of traditional clinical practice.

Generic 400 mg neurontin overnight delivery
Imaging Features Ultrasound features of acute pancreatitis ranges from normal findings to focal or diffuse enlargement of the gland with a heterogeneous pancreatic appearance or decrease of echogenicity (more hypoechoic than the liver) as a result of edema symptoms low blood sugar discount neurontin 800 mg with visa. In more severe cases, the presence of peripancreatic fluid collections, ascites, and occasionally vascular (B) necrosis and acute fluid collections. In the course of acute pancreatitis, pancreatic necrosis and peripancreatic fat necrosis undergo liquefaction, resulting in a mixed collection of fluid and necrotic debris. The most important disadvantages of ultrasound remain its operator dependence and limited visualization of the whole pancreas, especially the peripancreatic retroperitoneal region, due to body habitus, overlying bowel gas from an associated adynamic ileus, and the presence of monitoring lines or surgical drains or dressings. In more severe cases, pancreatic parenchymal necrosis develops, with acute fluid collections that occur mostly around the pancreas, in the anterior pararenal space, and in the lesser sac. The precontrast T1-weighted images demonstrate that most of the peripancreatic fluid is hemorrhagic in nature. Acute Pancreatitis 539 patients with an allergy to iodinated contrast material and in those with functional renal impairment. There is no convincing evidence that any intravenous iodinated contrast agent has detrimental effects on acute pancreatitis. Enlargement of the pancreatic gland can be depicted on any sequence, whereas parenchymal edema and hemorrhage are better delineated on unenhanced T1-weighted images. Axial T2-weighted image (B) demonstrates prominence of the dorsal pancreatic duct. The patient was stented and recovered completely with no need for additional interventions. Differential Diagnosis Acute abdominal conditions (biliary colic, acute bowel perforation, bowel obstruction, mesenteric ischemia, abdominal aortic aneurysm) may be confused clinically with acute pancreatitis; some of these may be associated with elevated serum amylase levels, but cross-sectional imaging features of these entities allow differentiation from acute pancreatitis. Autoimmune pancreatitis: Rarely presents with acute onset of pain and elevated pancreatic function tests. Imaging appearance of diffuse autoimmune pancreatitis, however, may present as a swollen pancreas with decreased signal and enhancement on T1-weighted images. Presence of a peripancreatic fibrous capsule, bile duct hyperenhancement, and lack of peripancreatic fluid collections suggests autoimmune pancreatitis. Pancreatic adenocarcinoma: Can cause acute pancreatitis due to duct obstruction and has to be excluded as the cause. Pancreatic and bile duct obstruction, presence of a mass lesion, and clinical features such as painless jaundice and weight loss may suggest underlying pancreatic cancer. Management/Clinical Issues the therapy of interstitial pancreatitis is supportive. Fluoroscopic image (B) obtained after transgastric puncture of the collection shows a guide wire in the collection and a balloon dilating the tract. Video endoscopic image (C) obtained during the debridement illustrates the extent of the collection and the tool used to remove the necrotic debris. Fluoroscopic image (D) obtained after the procedure shows several pigtail catheters bridging the stomach and the walled-off collection. Prognosis of interstitial pancreatitis is generally favorable with mortality below 3%. Mortality is usually due to unresolved organ failure, especially in a patient with comorbidity. Necrotizing pancreatitis is generally a more severe disease than interstitial pancreatitis. Of the patients who die, half die in the first 7 to 14 days because of persistent organ failure. The other half die later because of complications due to necrotizing pancreatitis, such as unresolved organ failure, development of infected necrosis, or some other complication of necrotizing pancreatitis. Clinical care in the first several weeks is dominated by fluid resuscitation, careful pulmonary care, and treatment of other clinical features such refractory shock, renal failure, and evolving pulmonary insufficiency, which may require intubation. Patients who will be without oral nourishment for several weeks should be started on nasojejunal feeding. The use of antibiotics should be restricted to those who have an infection such as pneumonia, urinary tract infection, or bloodstream infection. The role of endoscopic sphincterotomy in severe biliary pancreatitis remains somewhat controversial. In general, if there is strong suspicion of a retained stone in the common bile duct and persistent organ failure that could reflect ascending cholangitis, an endoscopic biliary sphincterotomy is recommended. Patients with infected necrosis require the use of appropriate antibiotics and debridement of the infected necrosis. The timing of debridement of the infected necrosis is dictated by the overall condition of the patient. In recent years, there has been consideration for delaying debridement until such time as the infectious process has evolved into walled-off necrosis. In necrotizing pancreatitis not associated with organ failure or other complications, mortality is less than 5%. The mortality of necrotizing pancreatitis in association with multisystem organ failure is, however, as high as 50%. Key Points Approximately 90% of cases of acute pancreatitis are interstitial (mortality approximately 3%); 10% are necrotizing pancreatitis (overall mortality approximately 15%). Severity of acute pancreatitis during hospitalization is defined primarily by persistent organ failure and pancreatic necrosis. Treatment of sterile necrosis is generally nonsurgical except for symptomatic walled-off necrosis; treatment of infected necrosis is surgical, endoscopic, and occasionally image-guided percutaneous debridement. Mortele Definition Chronic pancreatitis is a progressive, irreversible inflammatory and fibrosing disease of the pancreas with clinical manifestations of chronic abdominal pain, weight loss, and possible pancreatic exocrine and endocrine insufficiency. Demographic and Clinical Features Chronic pancreatitis should be in the differential diagnosis of any patient presenting with epigastric pain radiating to the back, steatorrhea, considerable weight loss, or recurrent acute pancreatitis. Patients generally have known risk factors for chronic pancreatitis, such as moderate to heavy alcohol or tobacco exposure. A long history of heavy alcohol consumption is the most common cause of chronic pancreatitis; other etiologies include hyperlipidemia, hyperparathyroidism, trauma, cystic fibrosis, autoimmune diseases, smoking, and pancreas divisum. In approximately 30% to 40% of these patients, chronic pancreatitis has no apparent underlying cause; it is therefore considered to be "idiopathic" chronic pancreatitis. Pathology the pathology of chronic pancreatitis varies with its etiology, but there are certain abnormalities that are present in all types. Typical gross changes include atrophy of the pancreas, dilation of ducts, loss of lobular pattern in areas of scarring, and the presence of stones in the ducts. Histologically the two most common features of chronic pancreatitis are loss of acinar tissue (atrophy) and fibrosis. The fibrosis typically surrounds the lobules (perilobular or interlobular fibrosis) or extends into the lobules of acinar tissue (intralobular fibrosis). Initially the scarring can be focal; and may then progress to diffusely involve the pancreas. The loss of acinar tissue may result in exocrine insufficiency and ultimately loss of islet tissue with development of diabetes. A chronic inflammatory infiltrate may be present, but this feature is highly variable and disappears late in the course of chronic pancreatitis. Imaging Features Trans-abdominal sonography is significantly limited in the diagnosis of chronic pancreatitis; it cannot be used to evaluate the complete pancreas in most patients owing to patient body habitus or overlying bowel gas. Moreover, the majority of the findings seen in chronic pancreatitis on ultrasound are neither sensitive nor specific for the diagnosis. Reported advanced findings on ultrasound include alterations in size and echogenicity of the gland, pancreatic calcifications, pancreatic duct dilatation and irregularity, biliary dilatation, and the presence of intra- and/ or extrapancreatic pseudocysts. Pancreatic calcifications are seen as multiple punctate hyperechoic foci that may or may not shadow and may show color Doppler "twinkling" artifact. The degree and pattern of pancreatic calcification may change over time and no correlation exists between exocrine function and the amount of pancreatic calcification. The pancreas may demonstrate a heterogeneous echotexture with mixed areas of hyper- and hypoechogenicity. The hyperechoic regions are likely related to fibrosis and calculi, while the hypoechoic regions are associated with areas of inflammation.

Buy neurontin with amex
We will focus on the effects of insulin on skeletal muscle treatment resistant anxiety buy generic neurontin 100mg, which is the tissue most studied in humans. The phosphorylation of Akt represents a critical step in insulin signaling, and is a branching point for signaling related to insulin-stimulated protein anabolism. In humans, these effects of insulin have been described in skeletal muscle [810]. In addition to the role of S6K1 in mediating translation initiation and elongation, phosphorylation of S6K1 negatively regulates insulin signaling. Activation of the insulin signaling cascade also reduces protein breakdown by stabilizing lysosomes and reducing the activity of the ubiquitin-proteasome pathway [21]. Collectively, these findings highlight that there is an intricate network of cellular regulatory pathways that, when stimulated by insulin, can increase initiation of translation and protein synthesis, and also reduce protein breakdown through the inhibition of the two major proteolytic systems. Effect of insulin on tissue amino acid availability Control of tissue amino acid availability is an additional mechanism that appears to be necessary for activation of protein anabolism by insulin. Under physiologic conditions, insulin exerts two main effects on amino acid availability. An increase in blood insulin concentration increases the amount of tissue that is exposed to amino acids via endothelium-dependent vasodilation. In turn, the stimulation of muscle perfusion increases the flux of amino acids delivered to the tissue. The increase in amino acid delivery can occur even in conditions of low blood amino acid concentrations, such as during insulin infusion in the fasting state, as long as the blood amino acid concentrations do not decrease below the lower normal value [7]. In other words, vasodilation and increased amino acid delivery are necessary but not sufficient mechanisms for insulin to stimulate protein synthesis and anabolism. This indicates that insulin signaling must also be activated in the target tissue to stimulate protein anabolism. This process is emerging as a major regulator of skeletal muscle protein homeostasis [29]. Kinetic studies using stable isotope methodologies have highlighted that hyperinsulinemia does indeed increase amino acid uptake from blood into skeletal muscle in healthy young humans, and that this process appears to be a limiting factor for the stimulatory effect of insulin on muscle protein synthesis [7]. Effects of insulin on protein metabolism in specific tissues the effects of insulin on protein metabolism vary in the different tissues and organs. In some tissues, insulin exerts its anabolic action primarily via increased protein synthesis, while in others its antiproteolytic effect prevails. We will discuss below the effects that insulin exerts on the tissues that contribute the most to protein metabolism at the whole body level: skeletal muscle, splanchnic tissues, and skin. Skeletal muscle Skeletal muscle contains approximately 50% of the body protein [1]. Insulin is an important anabolic hormone in humans because it physiologically increases net accretion of skeletal muscle proteins in healthy, young adults [7,30,31]. The net anabolic effect of insulin on skeletal muscle proteins has been unequivocally reported in the literature [7,3041]. However, the mechanisms by which insulin enhances muscle protein anabolism in humans have been the matter of intense discussion for more than two decades. Some of the studies reported a stimulatory effect of insulin on muscle protein synthesis [7,31,32,37,41,42], while others reported primarily a decrease in muscle protein breakdown [7,30,3336,40,41]. There are several possible explanations for these discrepancies between human studies. Muscle protein turnover is typically measured on the leg or the forearm with stable isotope tracer methodologies using the arteriovenous balance technique and/or the precursor-product method [43]. Selection of the sampling site, leg or forearm, is critical for the arteriovenous balance technique. This method implies the measurement of the balance of unlabeled amino acids and amino acid tracers across the selected limb, assuming that amino acid metabolism occurs mostly in the limb skeletal muscle. However, it is possible that the relative contribution of non-muscle tissues (skin, bone, bone marrow) to leg or forearm amino acid turnover is not the same. Bone marrow proteins are likely to turnover faster than muscle proteins because of the high cellular turnover, but no data are available on the effects of insulin on this tissue. Thus, the sampling site may have been partly responsible for some of the differences reported between studies. Selection of the arteriovenous balance model to calculate muscle protein turnover is even more important and can significantly affect the conclusions of the study. There are three fundamental models available to measure human muscle protein synthesis [43]: the two arteriovenous balance models, two-pool and three-pool, and the precursor-product model. The two-pool model estimates muscle protein synthesis and breakdown using the amino acid enrichments and concentrations in the artery and vein of the selected limb. These parameters are based on the extraction of the labeled amino acid from the artery, the appearance of unlabeled amino acid from the muscle into the vein, and the net arteriovenous amino acid difference [43]. Thus, this model provides data regarding the kinetics of blood amino acids across the leg or forearm, but cannot measure the intracellular recycling of those amino acids that are released from protein breakdown and re-incorporated into proteins without appearing in the blood. In other words, the two-pool arteriovenous method allows for the measurement of the effect of treatments on the net kinetics of blood amino acids across the selected limb, while not offering any insight into the actual intracellular amino acid kinetics. The three-pool model is an expansion of the two-pool model and relies not only on the measurement of amino acid enrichments and concentrations in the arterial and venous blood, but also on the direct measurement of the amino acid enrichments in the free tissue water. This allows for the direct measurement of the intracellular amino acid Protein metabolism in health and diabetes 255 kinetics, including recycling from breakdown to synthesis. In addition, it also allows for calculation of the amino acid transport rates from artery into the muscle tissue, and from the muscle tissue into the venous blood. Many of the human studies on the effects of insulin on muscle protein turnover have utilized the two-pool method [7,8,30,3340,45,46], but most recently several studies have utilized the three-pool method either alone [31] or in conjunction with the two-pool and/or the precursor product method [7,8,45,46]. In those studies in which more than one model was used the results of the two- and three-pool model tended to be consistent, while the results of the arteriovenous balance methods and the precursor-product method not always agreed possibly due to the different timing of the measurements [7]. Samples for the arteriovenous methods are in fact collected in a shorter time frame as compared to samples for the precursor-product method. The latter involves measurement of the incorporation rate of an amino acid tracer into muscle proteins and provides an integrated view of the overall effect of insulin on muscle protein synthesis over several hours. A second and, possibly, more important issue to be considered is differences in study design that may have differentially emphasized the complex mechanisms by which insulin exerts its action on muscle protein metabolism. Specifically, a review of the human studies on the effects of insulin on skeletal muscle protein turnover suggests that such conflicting results could be entirely explained by differences in amino acid availability during insulin infusion. The studies reporting an increase in skeletal muscle protein synthesis as the primary mechanism of the insulin-induced muscle protein anabolism also reported an increase in amino acid availability [7,31,32,3742]. Conversely, the studies that found a significant reduction in skeletal muscle protein breakdown with no significant changes in protein synthesis during hyperinsulinemia also reported a decrease or no change in amino acid availability [7,30,3336,41]. The differences in amino acid delivery and availability were due to differences in amino acid concentrations and blood flow. The changes in amino acid concentrations were determined by the modality of insulin infusion, and/or by the concomitant infusion of exogenous amino acids, or lack of thereof. Specifically, systemic insulin infusion dramatically decreases blood amino acid concentrations [30,40] unless amino acids are replaced by exogenous infusion [30,34,37,39,40,47,48]. Conversely, local insulin infusion in a leg or a forearm allows for the exposure of muscle tissue to relatively high insulin levels while avoiding a major reduction in blood amino acid concentration [31,33]. As discussed in detail earlier, a fundamental variable that significantly affects the response of skeletal muscle protein synthesis to insulin is blood flow. Under physiologic conditions of normal amino acid concentrations, insulin has been reported to enhance microvascular flow [26,49], amino acid delivery to the muscle, and amino acid transport into the myofibers [7,8,31], with a resulting stimulation of skeletal muscle protein synthesis [7,8,31]. Third, it is also important to highlight that skeletal muscle protein metabolism is less sensitive to insulin as compared to glucose or fat metabolism, so that the stimulatory effect of insulin on skeletal muscle protein synthesis occurs only at or beyond physiologic postprandial levels of insulin concentrations (approximately 50 U mL-1 or 300 pmol L-1) [7,31,37]. Lower insulin doses, such as doubling the basal insulin concentration (approximately 2025 U mL-1 or 120150 pmol L-1), have no effect on protein synthesis [7,46] and may [46] or may not [7] decrease muscle protein breakdown. Fourth, it is important to recognize that measures of skeletal muscle protein metabolism represent the combined result of the turnover of individual proteins. Animal studies have reported that insulin can differentially affect the synthesis of individual proteins in the skeletal muscle [50]. Future studies will have to explore the potential differential effects of insulin on individual skeletal muscle protein turnover. Splanchnic tissues Insulin may affect the protein turnover rates of splanchnic tissues, particularly the liver.
Order neurontin in united states online
A more direct proof of the relevance of an anticipated secretory response comes from a study evaluating the effects on glucose levels of two insulin profiles with the same mean value but different timing: the anticipated profile produces lower glucose than the delayed one [163] medicine 5000 increase buy discount neurontin on line. It is also likely that the potentiation phenomena observed during the 24 hours contribute to a more efficient control of glucose tolerance. The -cell response exhibits a high degree of sophistication and can finely tune insulin secretion to cope with the metabolic needs. Appropriate control of glucose levels, indispensable for healthy living, is almost entirely ensured by this small but powerful organ. As discussed in depth in Chapter 24, -cell dysfunction is a key feature of diabetes. Comprehension of the mechanisms controlling such a fine and articulated function is not straightforward, but gaining an understanding of the as yet unresolved questions in order to pave the way for new and more effective cures for this widespread disease is of prime importance. It should be recalled that two distinct mechanisms determine glucose sensitivity: the ability of the cell to respond to intravenous glucose and the incretin effect. Both these mechanisms contribute to glucose homeostasis in a significant manner [38,161]. American Journal of Physiology-Endocrinology and Metabolism 2002;283:E1159E1166. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. American Journal of Physiology- Endocrinology and Metabolism 2007;293:E17091715. However, in certain circumstances regulation of insulin and glucagon secretion is also dependent on the autonomic nerves which innervate the islets [1]. The islet nerves also innervate the islet blood vessels, and may therefore also affect islet function through changes in blood flow. Islet parasympathetic nerves the parasympathetic nerves innervating the islets originate in the pancreatic ganglia, whose activity is controlled by preganglionic nerves, originating in the dorsal motor nucleus of the brain [1]. In addition, the adjacent duodenum projects parasympathetic fibers to the pancreatic ganglia [2]. The preganglionic nerve fibers form classical nerve terminals and synapses within the ganglia where they release acetylcholine to activate nicotinic receptors on the postganglionic neurons. The nerves have small vesicles which contain acetylcholine and large dense core vesicles which contain neuropeptides. Both acetylcholine and neuropeptides are released from small varicosities in the nerves, from which they diffuse to the islet endocrine cells and activate specific receptors which cause stimulation of both insulin and glucagon secretion. A main physiologic importance of this regulation is the early phase Introduction the traditional view is that islet hormone secretion is mainly regulated by circulating nutrients (glucose, amino acids, free fatty acids) as well as the gut incretin hormones (glucagon-like International Textbook of Diabetes Mellitus, Fourth Edition. Another physiologic importance of the parasympathetic islet nerves may be, together with the sympathetic nerves, the stimulation of glucagon secretion during hypoglycemia [4]. The classical mechanism invoked to explain stimulation of insulin and glucagon secretion during vagal nerve activation is the release of the neurotransmitter acetylcholine. The released acetylcholine diffuses to the endocrine cells and activates muscarinic receptors (mainly the M1 and M3 subtypes of the muscarinic receptor) which results in stimulation of insulin and glucagon secretion [5]. Another mechanism is phosphorylation-/arrestin-dependent coupling of the muscarinic M3 receptor to protein kinase D1 [8]. The critical importance of the cholinergic mediation of islet hormone secretion for glucose homeostasis is evident from studies using mice with either -cell deletion of the M3 receptors or -cell overexpression of M3 receptors [8]. The -cell M3 knockout mice have defective insulin secretion and glucose intolerance, whereas -cell M3 receptor overexpressing mice have increased insulin secretion and enhanced glucose tolerance. The mice with -cell overexpression of M3 receptors are also resistant to diet-induced glucose intolerance and hyperglycemia [8]. Neuropeptides contained in islet parasympathetic nerve terminals may also contribute to vagally induced stimulation of insulin and glucagon secretion, since in the dog insulin secretion after vagal nerve activation is not abolished by atropine, which blocks the muscarinic receptors. However, it is abolished by hexamethonium, which blocks the nicotinic receptors needed to activate postganglionic nerves [9]. The neuropeptides localized to islet parasympathetic nerve terminals and therefore potential mediators of the islet noncholinergic effects are gastrin-releasing polypeptide, vasoactive intestinal polypeptide, and pituitary adenylate cyclase-activating polypeptide [9]. It consists of a 27-amino acid residue, which is -amidated in its C-terminal methionine. The peptide is highly conserved during evolution, having an identical N-terminal end to bombesin, and the human and porcine forms of the peptide differ in only two residues. It is also found in nerve terminals and their neuronal cell bodies in pancreatic ganglia, as demonstrated both in mice and rats [26]. These mice are viable, develop normally and have normal baseline glucose and insulin levels as well as normal pancreatic insulin content. It therefore mimics both the stimulation of insulin and glucagon secretion induced by parasympathetic nerve activation. Parasympathetic effects and mediation As reviewed earlier, activation of the parasympathetic nerves stimulates both insulin and glucagon secretion, and this may be of particular relevance for the cephalic and early meal phases of insulin secretion as well as for the glucagon response to hypoglycemia. In classical neurobiology, several criteria need to be met before a candidate neurotransmitter, such as these neuropeptides, can be proven to be a physiologic neurotransmitter [30] (Table 9. These criteria include that the potential neurotransmitter is localized to the nerves, is released by activation of the nerves, its effects mimic that of the nerves and that inhibition of its effect alters the responses to nerve activation. Islet sympathetic nerves Islets are densely innervated by sympathetic nerves, as demonstrated by fluorescence microscopy, electron microscopy, and immunocytochemistry [1]. Electrical activation of the sympathetic nerves inhibits insulin secretion and stimulates glucagon secretion [31]. However, hyperpolarization with closure of the voltage-sensitive calcium channels and a direct exocytotic action may also be involved. In addition to noradrenaline, neuropeptides also contribute to sympathetically induced inhibition of insulin secretion. First, combined 2- and -adrenoreceptor blockade by yohimbine and propranolol does not prevent the effect of sympathetic activation to inhibit basal insulin secretion. Second, exogenous noradrenaline does not mimic the effect of sympathetic activation to inhibit basal insulin secretion [31]. Two sympathetic neuropeptides, galanin and neuropeptide Y, may mediate these nonadrenergic effects because they (a) are localized to both the islet sympathetic nerve terminals and their neuronal cell bodies in the celiac ganglion, (b) are released from the pancreas in response to electrical activation of the sympathetic nerves, and (c) each inhibit insulin secretion as demonstrated both in vivo and in vitro [1]. Galanin Galanin was isolated from the porcine gastrointestinal tract in 1983 and named because of its glycine C-terminus and alanine N-terminus [32]. Galanin is a widely distributed neuropeptide both in the central and peripheral nervous systems. In islets, dense galanin innervation was first demonstrated in dogs, and later in other species, including humans [30,32]. However, species differences exist; for example, islet galanin innervation is more abundant in dogs and cats than in common laboratory animals like rats and mice [32]. In dogs, islet nerves that contain galanin also contain tyrosine hydroxylase as do their neuronal cell bodies in the celiac ganglion [30]. This hypothesis is supported by the substantial, although not total, loss of pancreatic galanin nerves that develops after chemical sympathectomy with 6-hydroxydopamine. Further evidence comes from the dog, whose pancreatic galanin is released after sympathetic nerve activation, and from the mouse, whose pancreatic galanin content is reduced by exercise stress [30]. Therefore, although galanin may occasionally also be found in certain other nerves, most data suggest that islet galanin is a sympathetic neuropeptide. Galanin has been demonstrated to potently inhibit basal and stimulated insulin secretion both in vivo, as demonstrated in dogs, rats, and mice, and in vitro, as shown in isolated rodent and human islets and in perfused rat pancreas. The actions of galanin have been shown to be transmitted by signals induced by activation of three different galanin receptor subtypes, called GalR1, GalR2, and GalR3, respectively. The GalR1 form is expressed in insulin-producing cells and therefore most likely the mediator of the inhibitory effect of galanin on insulin secretion [33]. Functional studies have demonstrated that galanin is released from the dog pancreas during sympathetic nerve activation in a quantity that is sufficient to mimic the inhibition of insulin secretion induced by sympathetic nerve stimulation [30]. These data therefore suggest that galanin 130 Chapter 9 helps mediate sympathetically induced inhibition of insulin secretion. A similar conclusion comes from a study in mice in which two minutes of swimming causes a 50% inhibition of glucose-stimulated insulin secretion [36]. When galanin was immunoneutralized in these mice by pretreatment with a high-titer galanin antiserum, swimming no longer inhibited glucose-stimulated insulin secretion, suggesting that galanin mediated this exercise-induced inhibition of insulin secretion. Further support comes from galanin knockout mice where the inhibitory effect of sympathetic nerve activation of insulin secretion is impaired [37]. Although a galanin receptor antagonist, galantide, exists [38], studies using this approach to prove a role for galanin in sympathetically mediated inhibition of insulin secretion remain to be performed. Sympathetic effects and mediation Sympathetic nerves inhibit insulin and stimulate glucagon secretion.


