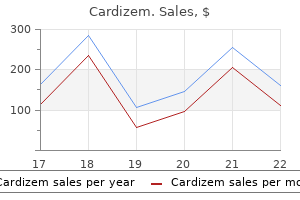
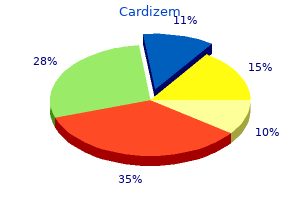
Cardizem 180 mg lowest price
Induction of anergy leaves T cells unresponsive blood pressure normal karne ka tarika order 180mg cardizem mastercard, even on subsequent exposure to antigen with sufficient costimulation. Autoimmunity: A Breach of Tolerance Several theories exist that aim to explain the origins of individual autoimmune disorders: Molecular Mimicry. The hypothesis of "molecular mimicry" reasons that unique pathogen-derived antigens resemble endogenous host antigens. Therefore, pathogen-induced disturbances to thymic events might negatively affect elimination of autoreactive T cells. Regardless of the mechanism, central tolerance has thus far not been exploited for pharmacological intervention. When an individual is reexposed to the hapten, antigen-specific T cells migrate to the skin, causing local inflammation and edema. Autoimmunity, Immune Deficiency, and Transplant Rejection Just as for a regular and, appropriate immune response, autoimmunity is founded in either humoral (autoantibody) or cellular (T-cell) responses. As described in the section on lymphocyte development, the process of central tolerance limits the development of autoreactive B and T cells. This process is imperfect, and mechanisms of peripheral tolerance are in place to limit the activity of self-reactive lymphocytes that manage to escape thymic deletion. Consequently, Immune Deficiencies Primary immunodeficiency encompasses genetic or developmental defects in the immune system that leave the individual susceptible to infections to various degrees. Severe forms (severe combined immunodeficiency) are typically diagnosed in early childhood and are associated with significantly reduced life expectancy. Presently, nine classes of primary immunodeficiency are recognized, totaling over 120 unique conditions. Unfortunately, current treatment options are limited to supportive therapy in the form of antiviral, antifungal, and antibacterial drugs. Current nomenclature incorporates information on the source of the antibody as well as the intended target tissue. Acquired immunodeficiency refers to the loss of immune function due to environmental exposure. These conditions encompass patients receiving immune-suppressive therapy for autoimmune disorders or to prevent transplant rejections. Acquired immunodeficiency is also commonly observed in patients suffering from hematopoietic malignancies, as tumor cells outcompete functional leukocytes for space in the bone marrow or blood. Immune checkpoints refer to inhibitory (often negative-feedback) pathways that limit the amplitude and duration of an immune response. Under normal physiological conditions, immune checkpoints protect tissues from damage during an immune response and contribute to the maintenance of self-tolerance. In conditions of chronic viral infections and cancers, chronic antigen persistence results in the development of dysfunctional "exhausted" T cells. These inhibitory pathways resulting in T-cell exhaustion have been documented in mice, monkeys, and humans, highlighting their importance in modulating T-cell function. Cancer cells express a variety of genetic and epigenetic alterations that distinguish them from their normal counterparts. These tumor-associated antigens can be recognized by the host immune system; antitumor T cells are generated, which then eliminate these transformed cells. One of these evasion strategies involves the manipulation of immune-inhibitory pathways or immune checkpoints. Tumors avoid being destroyed by actively stimulating these inhibitory receptors to turn off antitumor T cells. The intensity of rejection is minimized with increased compatibility between donor and recipient; however, a lifelong regimen of immunosuppressive drugs is unavoidable (see Chapter 35). Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. This older nomenclature, still in use by some workers, focused primarily on the source of the antibody (murine, human, chimeric, or humanized). The therapeutic goal is to interfere with this inhibitory interaction that is actively suppressing T cells in the tumor microenvironment. The tumor effectively inactivates the T cells and the tumor continues to grow (Pardoll, 2012; Tang et al. Immunotherapy to cancers holds great promise for treating patients with advanced disease, as evidenced by the success of clinical trials using this technology. Furthermore, combination strategies that include checkpoint blockade paired with radiation or chemotherapy may further increase responsiveness in cancer patients. One consequence of checkpoint blockade is that autoreactive T cells are also unleashed after therapy. Patients can develop toxicities that include hepatic, pneumonitis, colitis, rash, vitiligo, and endocrine pathology. Greater immunotherapy efficacy will likely be achieved when drugs are developed to target other inhibitory pathways and are used in combination, but caution must be evaluated to ensure patient safety (Callahan et al. Innate immunity turned insideout: antimicrobial defense by phagocyte extracellular traps. Four major classes of immunosuppressive drugs are discussed: glucocorticoids (see Chapter 46), calcineurin inhibitors, antiproliferative and antimetabolic agents (see Chapter 66), and antibodies. While there are similarities, the approach to the use of immunosuppressant drugs in transplant rejection has evolved separately from the approaches used to treat autoimmune disease and thus is presented separately. Immunosuppression Immunosuppressive drugs are used to dampen the immune response in organ transplantation and autoimmune disease. In transplantation, the major classes of immunosuppressive drugs used today are the following: Glucocorticoids Calcineurin inhibitors Antiproliferative/antimetabolic agents Biologicals (antibodies) the Immune Response the immune system evolved to discriminate self from nonself. Innate immunity (natural immunity) is primitive, does not require priming, and is of relatively low affinity, but it is broadly reactive. Adaptive immunity (learned immunity) is antigen specific, depends on antigen exposure or priming, and can be of very high affinity. The two arms of immunity work closely together, with the innate immune system most active early in an immune response and adaptive immunity becoming progressively dominant over time. B lymphocytes make antibodies; T lymphocytes function as helper, cytolytic, and regulatory (suppressor) cells. These cells not only are important in the normal immune response to infection and tumors but also mediate transplant rejection and autoimmunity. Immunoglobulins (antibodies) on the B-lymphocyte surface are receptors for a large variety of specific structural conformations. Once activated by specific antigen recognition, both B and T lymphocytes are triggered to differentiate and divide, leading to release of soluble mediators (cytokines, lymphokines) that perform as effectors and regulators of the immune response. Chapter 34 presents a more detailed view of the immune system at the levels of the molecules, cells, and organs involved in immunity. These drugs are successful in treating conditions such as acute immune rejection of organ transplants and autoimmune diseases. However, such therapies often require lifelong use and nonspecifically suppress the entire immune system, exposing patients in some instances to higher risks of infection and cancer. The calcineurin inhibitors and daily glucocorticoids, in particular, are nephrotoxic and diabetogenic, respectively, thus restricting their usefulness in a variety of clinical settings. Finally, newer small molecules and antibodies have expanded the arsenal of immunosuppressives. Thus, there are useful pharmacological tools that can substantially limit clonal expansion and potentially promote tolerance (Goldfarb-Rumyantzev et al. General Approach to Organ Transplantation Therapy Organ transplantation therapy is organized around five general principles. Employ multitier immunosuppressive therapy; simultaneously use several agents, each of which is directed at a different molecular target within the allograft response. Employ intensive induction and lower-dose maintenance drug protocols; greater immunosuppression is required to gain early engraftment or to treat established rejection than to maintain long-term immunosuppression. Investigation of each episode of transplant dysfunction is required, including evaluation for recurrence of the disease, rejection, drug toxicity, and infection (keeping in mind that these various problems can and often do coexist).
Purchase cardizem 180mg visa
Avermectins induce paralysis by activating a family of ligand-gated Cl- channels arteria palatina ascendens order genuine cardizem online, particularly glutamate-gated Cl- channels found only in invertebrates. Ivermectin probably binds to glutamate-activated Cl- channels found in nematode nerve or muscle cells and causes hyperpolarization by increasing intracellular chloride concentration, resulting in paralysis. Glutamate-gated Cl- channels probably are one of several sites of ivermectin action amongst invertebrates (Zufall et al. Lack of high-affinity avermectin receptors in cestodes and trematodes may explain why these helminths are not sensitive to ivermectin (Shoop et al. A 6-week regimen of doxycycline (100 mg daily), by killing the Wolbachia, leads to sterility of adult female Onchocerca worms. Virtually no ivermectin appears in human urine in either unchanged or conjugated form (Krishna and Klotz, 1993). Marked reduction of microfilariae in the skin results in major relief of the intense pruritus that is a feature of onchocerciasis. However, the drug is not curative because ivermectin has little effect on adult O. Annual doses of the drug are quite safe and substantially reduce transmission of this infection. Isolation of the anthelmintic components from cultures of this organism led to discovery of the avermectins, a novel class of 16-membered macrocyclic lactones (Campbell, 1989). Ivermectin (mectizan; stromectol; 22,23-dihydroavermectin B1a) is a semisynthetic analog of avermectin B1a (abamectin), an insecticide developed for crop management. Ivermectin now is used extensively to control and treat a broad spectrum of infections caused by parasitic nematodes (roundworms) and arthropods (insects, ticks, and mites) that plague livestock and domestic animals (Campbell, 1993). The duration of treatment is at least 5 years, based on the estimated fecundity of the adult worms. Ivermectin Chemistry Ivermectin exists as an odorless, off-white powder with high lipid solubility but poor solubility in water. It is a mixture of at least 80% 22,23-dihydroavermectin B1a and no more than 20% 22,23-dihydroavermectin B1b. Ivermectin, administered as a single dose of 150 to 200 g/kg, is the drug of choice for human strongyloidiasis (Marti et al. It is generally recommended that a second dose be administered a week following the first dose. Ivermectin is more effective in ascariasis and enterobiasis than in trichuriasis or hookworm infection. In the last two infections, although it is not curative, it significantly reduces the intensity of infection. Taken as a single 200-g/kg oral dose, ivermectin is a first-line drug for treatment of cutaneous larva migrans caused by dog or cat hookworms and is an option for treatment of scabies and head lice. In severe (crusted) scabies, ivermectin should be used in repeated doses, with one recommended regimen entailing seven doses of 200 g/kg given with food on days 1, 2, 8, 9, 15, 22, and 29. Adverse Effects; Drug Interactions Ivermectin is well tolerated by uninfected humans. In filarial infection, ivermectin therapy frequently causes a Mazzotti-like reaction to dying microfilariae. Rarely, more severe reactions occur that include high fever, tachycardia, hypotension, dizziness, headache, myalgia, arthralgia, diarrhea, and facial and peripheral edema; these may respond to glucocorticoid therapy. The drug can cause rare but serious side effects, occasionally resulting in permanent disability and encephalopathies in patients with heavy L. Loa encephalopathy is associated with ivermectin treatment of individuals with Loa microfilaremia levels 30,000 or more microfilariae per milliliter of blood. Praziquantel distributes throughout the body, with highest concentrations measured in the liver and kidneys. Breast milk concentrations are approximately 25% of plasma concentrations (Olliaro et al. In patients with severe liver disease, including those with hepatosplenic schistosomiasis, pharmacokinetic parameters might be altered (Olliaro et al. About 70% of an oral dose is recovered as metabolites in the urine within 24 h; most of the remainder is eliminated in the bile. Praziquantel is the drug of choice for treating schistosomiasis caused by all Schistosoma species that infect humans. The same three-dose regimen, used over 2 days, is highly effective against infections with the lung fluke Paragonimiasis westermani. Low doses of praziquantel can be used to treat intestinal infections with adult cestodes (a single oral dose of 25 mg/kg for Hymenolepis nana and 10 to 20 mg/kg for Diphyllobothrium latum, Taenia saginata, or T. Although albendazole is preferred for therapy of human cysticercosis, praziquantel represents an alternative agent; its use for this indication is hampered by the important pharmacokinetic interaction with dexamethasone and other corticosteroids that should be coadministered in this condition (Evans et al. This is principally due to concerns about the passage of the drug across the immature blood-brain barrier. Praziquantel Chemistry Praziquantel, a pyrazinoisoquinoline derivative, is a racemate, consisting of the biologically active enantiomer, R-praziquantel and the inactive distomer S-praziquantel. The white crystalline powder is slightly soluble in water and freely soluble in alcohol and in methylene chloride. Adverse Effects; Drug Interactions Mechanism of Action Praziquantel has two major effects on adult schistosomes. At the lowest effective concentrations, it causes increased muscular activity, followed by contraction and spastic paralysis. Affected worms detach from blood vessel walls and migrate from the mesenteric veins to the liver. At slightly higher concentrations, praziquantel causes tegumental damage and exposes a number of tegumental antigens (Redman et al. The clinical efficacy of this drug correlates better with tegumental action (Xiao et al. The drug is ineffective against juvenile schistosomes and therefore is relatively ineffective in early infection. An intact immune response is believed to be required for the clinical efficacy of the drug. It also promotes an influx of Ca2+ and possibly interacts with the variant Ca2+ channel Ca-var (Jeziorski and Greenberg, 2006), which is found in schistosomes and other praziquantel-sensitive parasites. However, Ca 2+ influx does not correlate with sensitivity to the drug (Pica-Mattoccia et al. Abdominal discomfort and drowsiness may occur shortly after taking praziquantel; these direct effects are transient and dose related. Indirect effects such as fever, pruritus, urticaria, rashes, arthralgia, and myalgia are noted occasionally. Such side effects and increases in eosinophilia often relate to parasite burden and may be a consequence of parasite killing and antigen release. In neurocysticercosis, inflammatory reactions to praziquantel may produce meningismus, seizures, and cerebrospinal fluid pleocytosis.
Order 120 mg cardizem
Increases in plasma osmolality above 290 mOsm/kg lead to an intense desire for water (thirst) hypertension and headaches order cardizem mastercard. Thus, the vasopressin system affords the organism longer thirst-free periods and, in the event that water is unavailable, allows the organism to survive longer periods of water deprivation. Above a plasma osmolality of approximately 290 mOsm/kg, plasma vasopressin levels exceed 5 pM. Since urinary concentration is maximal (1200 mOsm/kg) when vasopressin levels exceed 5 pM, further defense against hypertonicity depends entirely on water intake rather than on decreases in water loss. Lithium, which inhibits the renal effects of vasopressin, also enhances vasopressin secretion. Inhibitors of vasopressin secretion include ethanol, phenytoin, low doses of morphine, glucocorticoids, fluphenazine, haloperidol, promethazine, oxilorphan, and butorphanol. Cellular vasopressin effects are mediated mainly by interactions of the hormone with the three types of receptors: V1a, V1b, and V2. V1b receptors have a more limited distribution and are found in the anterior pituitary, several brain regions, pancreas, and adrenal medulla. An oral salt load activates hepatic por- tal osmoreceptors, leading to increased vasopressin release. This mechanism augments plasma vasopressin levels even before the oral salt load increases plasma osmolality. Vasopressin secretion is regulated hemodynamically by changes in effective blood volume or arterial blood pressure. Vasopressin is one of the most potent vasoconstrictors known, and the vasopressin response to hypovolemia or hypotension serves as a mechanism to stave off cardiovascular collapse during periods of severe blood loss or hypotension. Hemodynamic regulation of vasopressin secretion does not disrupt osmotic regulation; rather, hypovolemia/hypotension alters the set point and slope of the plasma osmolality-plasma vasopressin relationship. Neuronal pathways that mediate hemodynamic regulation of vasopressin release are different from those involved in osmoregulation. Baroreceptors in the left atrium, left ventricle, and pulmonary veins sense blood volume (filling pressures), and baroreceptors in the carotid sinus and aorta monitor arterial blood pressure. Vasopressin-synthesizing magno- cellular neurons have a large array of receptors on both perikarya and nerve terminals; therefore, vasopressin release can be accentuated or attenuated by chemical agents acting at both ends of the magnocellular neuron (Iovino et al. Because of these complexities, modulation of vasopressin secretion by most hormones or neurotransmitters is unclear. A number of drugs alter urine osmolality by stimulating or inhibiting vasopressin secretion. Stimulators of vasopressin secretion include Several sites of vasopressin action in kidney involve both V1 and V2 receptors. V1 receptors mediate contraction of mesangial cells in the glomerulus and contraction of vascular smooth muscle cells in vasa recta and efferent arterioles. V2 receptors mediate the most prominent response to vasopressin, which is increased water permeability of the collecting duct at concentrations as low as 50 fM. Activation of V1 receptors also causes influx of extracellular Ca2+ by an unknown mechanism. V2 receptors also increase Na+ transport in cortical collecting ducts, and this may synergize with aldosterone to enhance Na+ reabsorption during hypovolemia. Carbamazepine and chlorpropamide also enhance antidiuretic effects of vasopressin by unknown mechanisms. Lithium is of particular importance because of its use in the treatment of manic-depressive disorders (Kishore and Ecelbarger, 2013). Pharmacological Modification of the Antidiuretic Response to Vasopressin potency of vasopressin as a direct vasoconstrictor, vasopressin-induced pressor responses in vivo are minimal and occur only with vasopressin concentrations significantly higher than those required for maximal antidiuresis. To a large extent, this is due to circulating vasopressin actions on V1 receptors to inhibit sympathetic efferents and potentiate baroreflexes. Vasopressin helps to maintain arterial blood pressure during episodes of severe hypovolemia/hypotension. The effects of vasopressin on the heart (reduced cardiac output and heart rate) are largely indirect and result from coronary vasoconstriction, decreased coronary blood flow, and alterations in vagal and sympathetic tone. While vasopressin is not the principal corticotropin-releasing factor, vasopressin may provide for sustained activation of the hypothalamic-pituitary-adrenal axis during chronic stress. Studies in both laboratory animals and humans indicated that vasopressin and oxytocin are key regulators of social and emotional behaviors (Benarroch, 2013). Vasopressin is a potent vasoconstrictor (V1 receptor mediated), and resistance vessels throughout the circulation may be affected. However, because release of von Willebrand factor does not occur when desmopressin is applied directly to cultured endothelial cells or to isolated blood vessels, intermediate factors are likely to be involved. Vasopressin is stored in platelets, and activation of V1 receptors stimulates platelet aggregation. Aquaporins fold with transmembrane domains 1, 2, and 6 in close proximity and transmembrane domains 3, 4 and 5 in juxtaposition. Aquaporin 2, exclusively expressed in the principal cells of the connecting tubule and collecting duct, is the major vasopressin-regulated water channel. Aquaporin 3 and aquaporin 4 are expressed in the basolateral membranes of collecting duct principal cells and provide exit pathways for water reabsorbed apically by aquaporin 2. There are also a number of synthetic peptides with receptor-subtype specificity, and one nonpeptide agonist. Many vasopressin analogues were synthesized with the goal of increasing duration of action and selectivity for vasopressin receptor subtypes (V1 vs. V2 receptors, which mediate pressor responses and antidiuretic responses, respectively). Increasing V1 selectivity has proved more difficult than increasing V2 selectivity. Therefore, most of the available peptide vasopressin agonists and antagonists have some affinity for oxytocin receptors; at high doses, they may block or mimic the effects of oxytocin. Accumulation of mutant vasopressin precursor causes neuronal death, hence the dominant mode of inheritance. If polyuria is not controlled satisfactorily with chlorpropamide alone, addition of a thiazide diuretic to the regimen usually results in an adequate reduction in urine volume. Mutations in the V2 receptor gene may cause impaired routing of the V2 receptor to the cell surface, defective coupling of the receptor to G proteins, or decreased receptor affinity for vasopressin. These findings indicate that aquaporin 2 is essential for the antidiuretic effect of vasopressin in humans. Amiloride blocks Li+ uptake by the Na+ channel in the collecting duct system and may be effective in patients with mild-to-moderate concentrating defects. It is possible that the natriuretic action of thiazides and resulting extracellular fluid volume depletion play an important role in thiazide-induced antidiuresis. Moderate restriction of Na+ intake can enhance the antidiuretic effectiveness of thiazides. In a normal individual, an elevation in plasma vasopressin per se does not induce plasma hypotonicity because the person simply stops drinking owing to an osmotically induced aversion to fluids. Therefore, plasma hypotonicity only occurs when excessive fluid intake (oral or intravenous) accompanies inappropriate secretion of vasopressin. Although Li+ can inhibit the renal actions of vasopressin, it is effective in only a minority of patients, may induce irreversible renal damage when used chronically, and has a low therapeutic index. Only when symptomatic hypotonicity ensues, generally when plasma Na+ levels drop below 120 mM, should therapy with demeclocycline be initiated. Because hypotonicity, which causes an influx of water into cells with resulting cerebral swelling, is the cause of symptoms, the goal of therapy is simply to increase plasma osmolality toward normal. Because hypovolemia stimulates vasopressin release, patients may become hyponatremic owing to vasopressin-mediated retention of water.
Order genuine cardizem
These peptides contribute to inflammatory responses as autacoids that act locally to produce pain blood pressure xl cuff purchase cardizem online, vasodilation, and increased vascular permeability but can also have beneficial effects, for example, in the heart, kidney, and circulation (Bhoola et al. A number of serine proteases will generate kinins, but the two highly specific proteases that release bradykinin and kallidin from the kininogens are termed kallikreins. Plasma kallikrein and tissue kallikrein are distinct enzymes that are activated by different mechanisms (Bhoola et al. Human tissue kallikrein is 1 of 15 gene family members with high sequence identity that are clustered at chromosome 19q13. Tissue kallikrein is synthesized as a 29-kDa preproprotein in the epithelial cells or secretory cells in several tissues, including salivary glands, pancreas, prostate, and renal distal nephron (Bhoola et al. Tissue kallikrein also is expressed in human neutrophils; it acts locally near its sites of origin. The synthesis of tissue prokallikrein is controlled by a number of factors, including aldosterone in the kidney and salivary gland and androgens in certain other glands. Kallidin or [des-Arg10]-kallidin can be converted to the active peptides bradykinin or to [des-Arg9]-bradykinin by aminopeptidase cleavage of the N-terminal Lys residue. In a parallel fashion, the inactive decapeptide AngI is generated by the action of renin on the plasma substrate angiotensinogen. On smooth muscle cells, activation of kinin receptors coupling through Gq results in an increased [Ca2+]i and contraction. Kallikrein also plays a role in the intrinsic blood coagulation pathway (see Chapter 32). Plasma concentrations of bradykinin are difficult to measure because inadequate inhibition of kininogenases or kininases in the blood can lead to artifactual formation or degradation of bradykinin during blood collection. When care is taken to inhibit these processes, the reported physiological concentrations of bradykinin in blood are in the picomolar range. Carboxypeptidase N is expressed in the liver and constitutively secreted into the blood. The B2 receptor mediates the effects of bradykinin and kallidin under normal circumstances, whereas synthesis of the B1 receptor is induced by inflammatory conditions. Unlike the parent peptides, these kinin metabolites are potent ligands for B1 kinin receptors but not B2 kinin receptors. C-terminal des-Arg metabolites of bradykinin and kallidin produced by the actions of carboxypeptidases N and M. Interestingly, carboxypeptidase M and the B1 receptor interact on the cell surface to form an efficient signaling complex that enhances B1 receptor agonist affinity and can lead to allosteric activation of B1 receptor signaling by substrate binding to carboxypeptidase M (Zhang et al. B1 receptor expression is upregulated by tissue injury and inflammation and by cytokines, endotoxins, and growth factors. Carboxypeptidase M expression also is increased by cytokines, to such a degree that B1 receptor effects may predominate over B2 effects (Zhang et al. However, direct activation of B1 or B2 receptors on smooth muscle cells leads to coupling through Gq and increased [Ca2+]i, resulting in contraction. The B1 and B2 receptors differ in their time courses of downregulation after agonist stimulation; the B2 receptor response is rapidly desensitized, whereas the B1 response is not (Leeb-Lundberg et al. This likely is due to modification at a Ser/Thr-rich cluster present in the C-terminal tail of the B2 receptor that is not conserved in the B1 receptor sequence. However, the B1 receptor can heterodimerize with the B2 receptor and in this form can be cross desensitized by activation of the B2 receptor with agonist (Zhang et al. The kinins are powerful algesic agents that cause an intense burn- ing pain when applied to the exposed base of a blister. Although there is overlap, B2 receptors generally mediate acute bradykinin algesia, whereas the pain of chronic inflammation appears to involve increased numbers and activation of B1 receptors. Plasma kinins increase permeability in the microcirculation, acting on the small venules to cause disruption of the interendothelial junctions. Edema, coupled with stimulation of nerve endings, results in a wheal-and-flare response to intradermal injection. In acute attacks of hereditary angioedema, excess bradykinin is formed, as reflected by depletion of the upstream components of the kinin cascade, and is a primary mediator of swelling, laryngeal edema, and abdominal pain (Walford and Zuraw, 2014). Kinin levels are increased in a number of chronic inflammatory diseases and may be significant in gout, disseminated intravascular coagulation, inflammatory bowel disease, rheumatoid arthritis, or asthma. In addition, kinins and their receptors are associated with a variety of neuroinflammatory disorders, including neuropathic pain in diabetes, autoimmune encephalomyelitis, and Alzheimer disease. Kinins may contribute to the skeletal changes seen in chronic inflammatory states; kinins stimulate bone resorption through B1 and possibly B2 receptors, perhaps by osteoblastmediated osteoclast activation (see Chapter 44). The kinins have been implicated in allergic airway disorders such as asthma and rhinitis (Abraham et al. Inhalation of kinins causes bronchospasm mimicking an asthma attack in asthmatic patients but not in normal individuals. This bradykinin-induced bronchoconstriction is blocked by anticholinergic agents but not by antihistamines or cyclooxygenase inhibitors. Similarly, nasal challenge with bradykinin is followed by sneezing and glandular secretions in patients with allergic rhinitis, but not normal individuals or those with nonallergic, noninfectious perennial rhinitis. Functions and Pharmacology of Kallikreins and Kinins the utility of specific kinin receptor antagonists currently is being investigated in diverse areas such as pain, inflammation, chronic inflammatory Cardiovascular System. The endogenous kallikreinkinin system plays a minor role in the regulation of normal blood pressure, but it may be important in hypertensive states. Urinary kallikrein concentrations are decreased in individuals with high blood pressure. Bradykinin contributes to the beneficial effect of preconditioning to protect the heart against ischemia and reperfusion injury. Bradykinin also stimulates tissue plasminogen activator release from the vascular endothelium and may contribute to the endogenous defense against some cardiovascular events, such as myocardial infarction and stroke (Heitsch, 2003; Madeddu et al. Ecallantide is currently being investigated as a potential treatment of other forms of angioedema (Zuraw et al. The search is on to find a suitable stable B2 agonist for clinical evaluation that provides cardiovascular benefit without pro-inflammatory effects. Kallikrein is synthesized and secreted by the connecting cells of the distal nephron. Tissue kininogen and kinin receptors are present in the cells of the collecting duct. Bradykinin also causes natriuresis by inhibiting Na+ reabsorption at the cortical collecting duct. Kinins promote dilation of the fetal pulmonary artery, closure of the ductus arteriosus, and constriction of the umbilical vessels, all of which occur in the transition from fetal to neonatal circulation. Kinins and kinin receptor signaling have been associated with neuroinflammatory disorders, such as neuropathic pain in diabetes, autoimmune encephalomyelitis and Alzheimer disease. Kinin Receptor Antagonists the selective B2 receptor antagonist icatibant has been approved in the E. It is administered by a healthcare professional, or self-administered by the patient after training, at a dose of 30 mg in 3 mL of solution by subcutaneous injection in the abdomen. Additional doses may be administered at intervals of at least 6 h if the response is inadequate or symptoms recur, not to exceed three doses in any 24-h period. A common side effect experienced by most patients is a local reaction at the injection site. A small percentage of patients experienced fever, elevated transaminase, dizziness, nausea, headache, or rash. Other forms of angioedema are under investigation for responsiveness to icatibant. Kallikrein Inhibitors Aprotinin is a natural proteinase inhibitor that inhibits mediators of the inflammatory response, fibrinolysis, and thrombin generation following cardiopulmonary bypass surgery, including kallikrein and plasmin. Aprotinin was employed clinically to reduce blood loss in patients undergoing coronary artery bypass surgery, but unfavorable survival statistics in retrospective and prospective studies resulted in its discontinuation.
Discount 180mg cardizem overnight delivery
This derangement of carbohydrate metabolism leads to the accumulation of organic acids hypertension in african americans buy cardizem 60 mg lowest price, especially pyruvic, lactic, and acetoacetic acids. The same series of events also causes alterations of water and electrolyte balance. The low plasma Pco2 leads to decreased renal tubular reabsorption of bicarbonate and increased renal excretion of Na+, K+, and water. Water also is lost by salicylate-induced sweating (especially in the presence of hyperthermia) and hyperventilation. Because more water than electrolyte is lost through the lungs and by sweating, the dehydration is associated with hypernatremia. Toxic doses of salicylates lead to an exaggeration of the unfavorable cardiovascular responses seen at high therapeutic doses, and central vasomotor paralysis occurs. Large doses of salicylates may cause hyperglycemia and glycosuria and deplete liver and muscle glycogen; these effects are partly explained by the release of epinephrine. Such doses also reduce aerobic metabolism of glucose, increase glucose-6-phosphatase activity, and promote the secretion of glucocorticoids. There is a greater risk of hypoglycemia and subsequent permanent brain injury in children. Salicylates in toxic doses cause a significant negative nitrogen balance, characterized by an aminoaciduria. Adrenocortical activation may contribute to the negative nitrogen balance by enhancing protein catabolism. The combination of these effects leads to increased entry and enhanced oxidation of fatty acids in muscle, liver, and other tissues and to decreased plasma concentrations of free fatty acids, phospholipid, and cholesterol; the oxidation of ketone bodies also is increased. Salicylate poisoning represents an acute medical emergency, and death may result despite maximal therapy. Important adverse interactions of aspirin with warfarin, sulfonylureas, and methotrexate were mentioned previously (in Drug Interactions). Magnesiumaluminum hydroxide antacids can alkalize the urine enough to increase salicylic acid clearance significantly and reduce steady-state concentrations. Conversely, discontinuation of antacid therapy can increase plasma concentrations to toxic levels. Acetaminophen is suitable for analgesic or antipyretic uses; it is particularly valuable for patients in whom aspirin is contraindicated. Particular attention is warranted due to the availability of a wide variety of prescription and nonprescription multi-ingredient medications that represent potentially toxic overlapping sources of acetaminophen. Therapeutic doses of acetaminophen have no clinically relevant effects on the cardiovascular and respiratory systems, platelets, or coagulation. Rash and other allergic reactions occur occasionally, but sometimes these are more serious and may be accompanied by drug fever and mucosal lesions. Patients who show hypersensitivity reactions to the salicylates only rarely exhibit sensitivity to acetaminophen. The most serious acute adverse effect of overdosage of acetaminophen is a potentially fatal hepatic necrosis (Graham et al. Symptoms that occur during the first 2 days of acute poisoning by acetaminophen reflect gastric distress. Biopsy of the liver reveals centrilobular necrosis with sparing of the periportal area. In nonfatal cases, the hepatic lesions are reversible over a period of weeks or months. Adverse Effects and Toxicity Acetaminophen Acetaminophen (paracetamol; N-acetyl-p-aminophenol) is the active metabolite of phenacetin. Acetaminophen is available without a prescription and is used as a common household analgesic by children and adults. It also is available in fixed-dose combinations containing narcotic and nonnarcotic analgesics (including aspirin and other salicylates), barbiturates, caffeine, vascular headache remedies, sleep aids, toothache remedies, antihistamines, antitussives, decongestants, expectorants, cold and flu preparations, and sore throat treatments. Mechanism of Action Acetaminophen has analgesic and antipyretic effects similar to those of aspirin, but only weak anti-inflammatory effects. Severe liver damage occurs in 90% of patients with plasma concentrations of acetaminophen greater than 300 g/mL at 4 h or 45 g/mL at 15 h after the ingestion of the drug. This includes management of hepatic and renal failure, if they occur, and intubation if the patient becomes obtunded. Hypoglycemia can result from liver failure, and plasma glucose should be monitored closely. Indomethacin is a methylated indole derivative indicated for the treatment of moderate-to-severe rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis; acute gouty arthritis; and acute painful shoulder. Although indomethacin is still used clinically, mainly as a steroid-sparing agent, toxicity and the availability of safer alternatives have limited its use. Indomethacin has prominent anti-inflammatory and analgesic-antipyretic properties similar to those of the salicylates. There is a substantial first-pass effect, such that only about 50% of diclofenac is available systemically. The drug accumulates in synovial fluid after oral administration, which may explain why its duration of therapeutic effect is considerably longer than its plasma t1/2. There is enterohepatic cycling of the indomethacin metabolites and probably of indomethacin itself. The t1/2 in plasma is variable, perhaps because of enterohepatic cycling, but averages about 2. An intravenous formulation of indomethacin is approved for closure of persistent patent ductus arteriosus in premature infants. The principal limitation of treating neonates is renal toxicity, and therapy is interrupted if the output of urine falls significantly (<0. An injectable formulation of ibuprofen is an alternative for the treatment of patent ductus arteriosus. For acute pain such as migraine, a powdered form for dissolution in water and a solution for intravenous injection are available. A 1% topical gel, a topical solution, and a transdermal patch are available for short-term treatment of pain due to minor strains, sprains, and bruises. In addition, an ophthalmic solution of diclofenac is available for treatment of postoperative inflammation following cataract extraction and for the temporary relief of pain and photophobia in patients undergoing corneal refractive surgery. Hypersensitivity reactions have occurred following topical application and systemic administration. Elevation of hepatic transaminases in plasma by more than three times the upper normal limit, indicating significant liver damage, occurs in about 4% of patients (Rostom et al. Transaminases should be monitored during the first 8 weeks of therapy with diclofenac. Diarrhea may occur and sometimes is associated with ulcerative lesions of the bowel. Acute pancreatitis has been reported, as have rare, but potentially fatal, cases of hepatitis.
Order cardizem 60mg with mastercard
As with all anticoagulants blood pressure medication for migraines generic 60 mg cardizem fast delivery, the major adverse effect Designed as a decoy for the oral factor Xa inhibitors, andexanet alfa is a recombinant analogue of factor Xa that has the active site serine residue replaced with an alanine residue to eliminate catalytic activity and the Gla domain removed to preclude its incorporation in the prothrombinase complex. Higher doses of andexanet are needed to reverse rivaroxaban or edoxaban than apixaban. An ongoing phase 3 study is evaluating the effect of andexanet in patients taking these agents who present with serious bleeding. Rates of intracranial bleeding with rivaroxaban, apixaban, and edoxaban are at least 50% lower than that with warfarin. In healthy volunteers given edoxaban, an intravenous bolus of ciraparantag restored the whole-blood clotting time to normal. Inhibitors of Fibrinolysis -Aminocaproic Acid and Tranexamic Acid -Aminocaproic acid and tranexamic acid are lysine analogues that compete for lysine binding sites on plasminogen and plasmin, thereby blocking their interaction with fibrin. Therefore, these agents inhibit fibrinolysis and can reverse states that are associated with excessive fibrinolysis. The main problem with their use is that thrombi that form during treatment are not degraded. For example, in patients with hematuria, ureteral obstruction by clots may lead to renal failure after treatment with -aminocaproic acid or tranexamic acid. It is also used to reduce operative bleeding in patients undergoing hip or knee arthroplasty or cardiac surgery. Tranexamic acid is excreted in the urine; therefore, dose reduction is necessary in patients with renal impairment. Oral tranexamic acid is approved for treatment of heavy menstrual bleeding, usually given at a dose of 1 g four times daily for 4 days. Tissue Plasminogen Activator Tissue plasminogen activator is a serine protease and a poor plasminogen activator in the absence of fibrin. Intracranial hemorrhage is the most serious problem and can occur in up to 1% of patients. Antiplatelet Drugs Platelet aggregates form the initial hemostatic plug at sites of vascular injury. Platelets also contribute to the pathological thrombi that lead to myocardial infarction, stroke, and peripheral arterial thrombosis. Therefore, aspirin is maximally effective as an antithrombotic agent at doses much lower than those required for other actions of the drug. Higher doses do not improve efficacy and potentially are less efficacious because of inhibition of prostacyclin production, which can be largely spared by using lower doses of aspirin. Therefore, daily aspirin doses of 100 mg or less are used for most indications (Cohen et al. This effect is mediated by inhibition of phosphodiesterase or by blockade of uptake of adenosine, thereby increasing the dwell time of adenosine at cell surface adenosine A2 receptors that link to the stimulation of platelet adenylyl cyclase. Dipyridamole is a vasodilator that, in combination with warfarin, inhibits embolization Plaque disruption Therapeutic Uses. Clopidogrel is often used in combination with aspirin after coronary stent implantation. Clopidogrel increases the risk of bleeding, particularly when combined with aspirin or an anticoagulant. Reduced TxA2 release attenuates platelet activation and recruitment to the site of vascular injury. The newest member of the thienopyridine class, prasugrel is a prodrug that requires metabolic activation in the liver. It is hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite in the liver. Virtually all of the absorbed prasugrel undergoes activation; by comparison, only 15% of absorbed clopidogrel undergoes metabolic activation. Because the active metabolites of prasugrel bind irreversibly to the P2Y12 receptor, its effect lasts the lifetime of the platelets. Moderate renal or hepatic impairment does not appear to change the drug pharmacodynamics. Dipyridamole is approved for secondary prevention of stroke when it is combined with low-dose aspirin. P2Y12 Receptor Antagonists Clopidogrel Clopidogrel is a thienopyridine prodrug that inhibits the P2Y12 receptor. It has largely replaced ticlopidine because clopidogrel is more potent and less toxic, with thrombocytopenia and leukopenia occurring only rarely. Inhibition of platelet activation is seen 2 h postingestion of a loading dose of clopidogrel, and platelets are affected for the remainder of their life span. The incidence of cardiovascular death, myocardial infarction, and stroke is significantly lower with prasugrel than with clopidogrel, mainly reflecting a reduction in the incidence of nonfatal myocardial infarction. The incidence of stent thrombosis is also lower with prasugrel than with clopidogrel. Prasugrel is associated with higher rates of fatal and life-threatening bleeding than clopidogrel. Because patients with a history of a prior stroke or transient ischemic attack are at particularly high risk of intracranial bleeding, the drug is contraindicated in such patients. Patients over 75 years of age should not be prescribed prasugrel because of the increased bleeding risk. If patients present with serious bleeding, platelet transfusion may be beneficial. Concomitant administration of prasugrel with an anticoagulant or nonsteroidal anti-inflammatory drugs increases the risk of bleeding. It can be given as a whole tablet or crushed in water and administered via a nasogastric tube. In contrast to prasugrel in patients with acute coronary syndrome, which is only indicated in those undergoing percutaneous intervention, ticagrelor is indicated in those undergoing intervention and in those managed medically. Ticagrelor is associated with a higher risk of intracranial bleeding than clopidogrel and is contraindicated in patients with a history of prior intracranial bleeding. Platelet transfusion is ineffective in patients taking ticagrelor who present with serious bleeding, and a neutralizing antibody is under investigation for urgent reversal. It is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction or peripheral artery disease (Arif et al. Vorapaxar increases the risk of bleeding and is contraindicated in patients with a history of intracranial bleeding, stroke, or transient ischemic attack. Concomitant aspirin at a dose greater than 100 mg daily may reduce the effectiveness of ticagrelor. Ticagrelor increases serum concentrations of simvastatin and lovastatin and may affect digoxin metabolism. The use of these agents has decreased with the availability of potent P2Y12 inhibitors such as prasugrel and ticagrelor. Cangrelor has a short half-life because it is rapidly dephosphorylated in the circulation to an inactive metabolite. The risk of bleeding with cangrelor is greater than that with clopidogrel during the coronary intervention. Administration of ticagrelor, prasugrel, or clopidogrel should be delayed until the cangrelor infusion is stopped. It also binds to the vitronectin receptor on platelets, vascular endothelial cells, and smooth muscle cells. The antibody is administered to patients undergoing percutaneous coronary intervention and, when used in conjunction with aspirin and heparin, has been shown to prevent recurrent myocardial infarction and death (Gara et al.
Order cardizem 60mg with visa
Therapeutic effectiveness is best measured by the resulting increase in the rate of production of red cells blood pressure solution buy cardizem 120mg otc. The magnitude of the marrow response to iron therapy is proportional to the severity of the anemia (level of erythropoietin stimulation) and the amount of iron delivered to marrow precursors. The small intestine regulates absorption and, with increasing doses of oral iron, limits the entry of iron into the bloodstream. Clinically, the effectiveness of iron therapy is best evaluated by tracking the reticulocyte response and the rise in the hemoglobin or the hematocrit. A full laboratory evaluation should be conducted, and poor compliance by the patient or the presence of a concurrent inflammatory disease must be explored. Once a response to oral iron is demonstrated, therapy should be continued until the hemoglobin returns to normal. This may require a considerable period of time because the rate of absorption of iron by the intestine will decrease markedly as iron stores are reconstituted. The prophylactic use of oral iron should be reserved for patients at high risk, including pregnant women, women with excessive menstrual blood loss, and infants. Iron supplements also may be of value for rapidly growing infants who are consuming substandard diets and for adults with a recognized cause of chronic blood loss. Except for infants, in whom the use of supplemented Orally administered ferrous sulfate is the treatment of choice for iron deficiency. Variations in the particular ferrous salt have relatively little effect on bioavailability; the sulfate, fumarate, succinate, gluconate, aspartate, other ferrous salts, and polysaccharide-ferrihydrite complex are absorbed to approximately the same extent. Reduced iron (metallic iron, elemental iron) is as effective as ferrous sulfate, provided that the material employed has a small particle size. Large-particle ferrum reductum and iron phosphate salts have much lower bioavailability. Ferric edetate has been shown to have good bioavailability and to have advantages for maintenance of the normal appearance and taste of food. It also is essential that the coating of the tablet dissolve rapidly in the stomach. Delayedrelease preparations are available, but absorption from such preparations varies. Ascorbic acid (200 mg) increases the absorption of medicinal iron by at least 30%. However, the increased uptake is associated with an increase in the incidence of side effects. When the object is the prevention of iron deficiency in pregnant women, for example, doses of 15 to 30 mg of iron per day are adequate. For a rapid response or to counteract continued bleeding, as much as 120 mg of iron may be administered four times a day. Thus, an individual with a hemoglobin of 50 g per liter may achieve a normal complement of 150 g/L in about 50 days, whereas an individual with a hemoglobin of 100 g/L may take only half that time. Much of the strategy of continued therapy depends on the estimated future iron balance. Patients with an inadequate diet may require continued therapy with low doses of iron. If the bleeding has stopped, no further therapy is required after the hemoglobin has returned to normal. Side effects of oral iron preparations include heartburn, nausea, upper gastric discomfort, and diarrhea or constipation. Only individuals with underlying disorders that augment the absorption of iron run the hazard of developing iron overload (hemochromatosis). Signs and symptoms of severe poisoning may occur within 30 min after ingestion or may be delayed for several hours. They include abdominal pain, diarrhea, or vomiting of brown or bloody stomach contents containing pills. Of particular concern are pallor or cyanosis, lassitude, drowsiness, hyperventilation due to acidosis, and cardiovascular collapse. The corrosive injury to the stomach may result in pyloric stenosis or gastric scarring. In the evaluation of a child thought to have ingested iron, a color test for iron in the gastric contents and determination of the concentration of iron in plasma can be performed. However, vomiting should be induced when there is iron in the stomach, and an X-ray should be taken to evaluate the number of pills remaining in the small bowel (iron tablets are radiopaque). When the plasma concentration of iron is greater than the total iron-binding capacity (63 mol; 3. With early treatment, the mortality from iron poisoning can be reduced from 45% to about 1%. Therapy With Parenteral Iron When oral iron therapy fails, parenteral iron administration may be an effective alternative. Parenteral iron can be given to iron-deficient patients and pregnant women to create iron stores, something that would take months to achieve by the oral route. The indications for parenteral iron therapy include documented iron deficiency and intolerance or irresponsiveness to oral iron. The rate of hemoglobin response is determined by the balance between the severity of the anemia (the level of erythropoietin stimulus) and the delivery of iron to the marrow from iron absorption and iron stores. Parenteral iron therapy should be used only when clearly indicated because acute hypersensitivity, including anaphylactic and anaphylactoid reactions, can occur. Other reactions to intravenous iron include headache, malaise, fever, generalized lymphadenopathy, arthralgias, urticaria, and, in some patients with rheumatoid arthritis, exacerbation of the disease. These include iron dextran, sodium ferric gluconate, ferumoxytol, iron sucrose, and ferric carboxymaltose. Ferumoxytol is a semisynthetic carbohydrate-coated superparamagnetic iron oxide nanoparticle approved for treatment of iron deficiency anemia in patients with chronic kidney disease; the ferumoxytol has to be administered safely as a 1. Indications for ferric gluconate and iron sucrose are limited to patients with chronic kidney disease and documented iron deficiency, although broader applications are being advocated (Larson and Coyne, 2014). Iron dextran can be administered by intravenous (preferred) or intramuscular injection. Given intravenously in a dose less than 500 mg, the iron dextran complex is cleared with a plasma t1/2 of 6 h. Intramuscular injection of iron dextran should be initiated only after a test dose of 0. However, local reactions and the concern about malignant change at the site of injection make intramuscular administration inappropriate except when the intravenous route is inaccessible. The patient should be observed for signs of immediate anaphylaxis and for an hour after injection for any signs of vascular instability or hypersensitivity, including respiratory distress, hypotension, tachycardia, or back or chest pain. Delayed hypersensitivity reactions also are observed, especially in patients with rheumatoid arthritis or a history of allergies.
Discount 60mg cardizem
These agents are effective in protecting against various asthma triggers hypertension 160100 buy 60 mg cardizem, such as exercise, cold air, and allergens. The nebulized route of administration is easier and safer than intravenous administration and just as effective. Inhalation is preferable to oral administration because systemic side effects are less. Differences in 2 receptor selectivity have been claimed but are not clinically important. Drugs in clinical use include albuterol (salbutamol), levalbuterol, metaproterenol, terbutaline, pirbuterol, as well as several not available in the U. Anti-inflammatory Effects Whether 2 agonists have anti-inflammatory effects in asthma is controversial. The inhibitory effects of 2 agonists on mast cell mediator release and microvascular leakage are clearly anti-inflammatory, suggesting that 2 agonists may modify acute inflammation. Tolerance to the bronchodilator effect of formoterol and the bronchoprotective effects of formoterol and salmeterol have been demonstrated but is of doubtful clinical significance. Although both formoterol and salmeterol have a similar duration of effect in clinical studies, there are differences. Formoterol has a more rapid onset of action and is an almostfull agonist, whereas salmeterol is a partial agonist with a slower onset of action. These differences might confer a theoretical advantage for formoterol in more severe asthma, whereas it may also make it more likely to induce tolerance. However, no significant clinical differences between salmeterol and formoterol have been found in the treatment of patients with severe asthma (Nightingale et al. This is due to 2 receptor stimulation of potassium entry into skeletal muscle, which may be secondary to a rise in insulin secretion. Hypokalemia might be serious in the presence of hypoxia, as in acute asthma, when there may be a predisposition to cardiac arrhythmias (Chapter 30). Combination inhalers are now the preferred therapy for patients with persistent asthma. Continuous treatment with an agonist often leads to toler- R- and inactive S-isomers. Although R-albuterol (levalbuterol) was more potent than racemic R/S-albuterol in some studies, careful dose responses showed no advantage in terms of efficacy and no evidence that the S-albuterol is detrimental in asthmatic patients (Lotvall et al. Because levalbuterol is usually more expensive than normally used racemic albuterol, this therapy has no clear clinical advantage. Side effects ance, which may be due to downregulation of the receptor (Chapter 12). In asthmatic patients, tolerance to the bronchodilator effects of 2 agonists has not usually been found. However, tolerance develops to the bronchoprotective effects of 2 agonists, and this is more marked with indirect bronchoconstrictors that activate mast cells. The reason for the relative resistance of airway smooth muscle 2 responses to desensitization remains uncertain but may reflect the large receptor reserve: More than 90% of 2 receptors may be lost without any reduction in the relaxation response. Experimental studies have shown that corticosteroids prevent the development of tolerance in airway smooth muscle and prevent and reverse the fall in pulmonary receptor density (Mak et al. Because of a possible relationship between adren- ergic drug therapy and the rise in asthma deaths in several countries during the early 1960s, doubts were cast on the long-term safety of agonists. A particular 2 agonist, fenoterol, was linked to the rise in asthma deaths in New Zealand in the early 1990s because significantly more of the fatal cases were prescribed fenoterol than the case-matched control patients (Beasley et al. An epidemiological study examining the links between drugs prescribed for asthma and death or near death from asthma attacks found a marked increase in the risk of death with high doses of all inhaled 2 agonists. The risk was greater with fenoterol, but when the dose is adjusted to the equivalent dose for albuterol, there is no significant difference in the risk for these two drugs. The link between high 2 agonist usage and increased asthma mortality does not prove a causal association because patients with more severe and poorly controlled asthma, who are more likely to have an increased risk of fatal attacks, are more likely to be using higher doses of 2 agonist inhalers and less likely to be using effective anti-inflammatory treatment. Indeed, in the patients who used regular inhaled steroids, there was a significant reduction in risk of death. Regular use of inhaled 2 agonists has also been linked to increased asthma morbidity. Regular use of fenoterol was associated with worse asthma control and a small increase in airway hyperresponsiveness compared with patients using fenoterol "on demand" for symptom control over a 6-month period (Sears, 2002). There is some evidence that regular inhaled 2 agonists may increase allergen-induced asthma and sputum eosinophilia (Gauvreau et al. No major adverse effects were reported in several large and prolonged studies and no evidence of cardiovascular problems (Kew et al. Many salts of theophylline have also been marketed; the most common is aminophylline. Antagonism of A1 receptors may be responsible for serious side effects, including cardiac arrhythmias and seizures. This is associated with a reduction in the antiapoptotic protein Bcl-2 (Chung et al. Future Developments the agonists will continue to be the bronchodilators of choice for asthma because they are effective in all patients and have few or no side effects when used in low doses. Use of large doses of inhaled 2 agonists indicates poor asthma control; such patients should be assessed and appropriate controller medication used. There is little advantage to be gained by improving 2 receptor selectivity because most of the side effects of these agents are due to 2 receptor stimulation (muscle tremor, tachycardia, hypokalemia). Methylxanthines Methylxanthines, such as theophylline, which are related to caffeine, have been used in the treatment of asthma since 1930, and theophylline is still widely used in developing countries because it is inexpensive. Theophylline became more useful with the introduction of reliable slow-release preparations. There is increasing evidence that theophylline has anti-inflammatory effects in asthma (Barnes, 2013c). Several sustained-release preparations are now available that are absorbed at a constant rate and provide steady plasma concentrations over a 12- to 24-h period. Both slow-release aminophylline and theophylline are available and are equally effective (although the ethylene diamine component of aminophylline has been implicated in allergic reactions). For nocturnal asthma, a single dose of slow-release theophylline at night is often effective. Once optimal doses have been determined, routine monitoring of plasma concentrations is usually not necessary unless a change in clearance is suspected or evidence of toxicity emerges. The dose of theophylline required to give these therapeutic concentrations varies among subjects, largely because of differences in clearance of the drug. In addition, there may be differences in bronchodilator response to theophylline; furthermore, with acute bronchoconstriction, higher concentrations may be required to produce bronchodilation. Theophylline is rapidly and completely absorbed, but there are large interindividual variations in clearance due to differences in hepatic metabolism. Because of these variations in clearance, individualization of theophylline dosage is required, and plasma concentrations should be measured 4 h after the last dose with slow-release preparations when steady state has been achieved. There is no significant circadian variation in theophylline metabolism, although there may be delayed absorption at night related to supine posture. In patients with acute asthma, intravenous aminophylline is less effective than nebulized 2 agonists and should therefore be reserved for those patients who fail to respond to , or are intolerant of, agonists. Theophylline should not be added routinely to nebulized 2 agonists because it does not increase the bronchodilator response and may increase their side effects. If the patient is already taking theophylline, or there are any factors that decrease clearance, these doses should be halved and the plasma level checked more frequently.