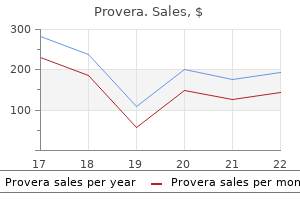
Provera 10mg free shipping
Regional release and removal of catecholamines and extraneuronal metabolism to metanephrines menstruation vs estrous order provera online. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Reference intervals for plasma free metanephrines with an age adjustment for normetanephrine for optimized laboratory testing of phaeochromocytoma. Sources and significance of plasma levels of catechols and their metabolites in humans. Measurement of plasma free metanephrine and normetanephrine by liquid chromatography-tandem mass spectrometry for diagnosis of pheochromocytoma. Pathophysiology and diagnosis of disorders of the adrenal medulla: focus on pheochromocytoma. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. Seasonal variation in plasma free normetanephrine concentrations: implications for biochemical diagnosis of pheochromocytoma. Adrenergic Mechanisms Peter W de Leeuw and Abraham A Kroon, Maastricht University Medical Center, Maastricht, the Netherlands r 2018 Elsevier Inc. Glossary Glucosensitive, thermosensitive, and barosensitive fibers these words refer to sympathetic nerve fibers that respond to alterations in blood glucose, temperature and blood pressure respectively. It is a classical control system with an input function, provided by afferents from various parts of the body, a central processor and output responses mediated by parasympathetic and sympathetic fibers as well as the adrenal medulla. Parasympathetic fibers can be afferent and efferent; sympathetic fibers are always efferent. Functionally, these neurons show some differentiation in the sense that when the sympathetic system is activated, this does not always produce the same effector response but one which is required to restore the primary functional disturbance. Acetylcholine is the neurotransmitter at preganglionic sites in both sympathetic and efferent parasympathetic fibers. It is also the neurotransmitter in postganglionic parasympathetic neurons and at the interface between sympathetic fibers and the adrenal medulla. At the level of the target organs the neurotransmitters react with specific receptors which can be classified as alpha-1, alpha-2, beta-1, beta-2 and beta-3 adrenoceptors. The A2/C2 neurons are innervated by primary visceroceptive afferents carried by the vagal and the glossopharyngeal nerves that convey information from mechano- and chemoreceptors related to cardiovascular, pulmonary, and gastrointestinal systems. Descending projections from C1 cells to the spinal cord innervate sympathetic preganglionic neurons and so can directly activate catecholamine-mediated responses to visceral changes. Ascending projections from A1/C1 and A2/C2 cells travel through the ventral adrenergic bundle and terminate in several limbic nuclei and hypothalamic areas. Neurons in these nuclei (particularly in the paraventricular and lateral hypothalamic area) are in turn connected to (1) the pituitary. Abundant evidence from experimental studies has shown that several adrenergic cell types are sensitive to local changes in glucose levels with a fall in glucose provoking eating and a rise in glucose. These neurons, therefore, are in a perfect position to mediate metabolic and behavioral responses to central (or systemic) changes in glucose levels and/or energy status. A1/C1 and A2/C2 cells are also activated by other stressful situations such as hemorrhage, immune challenge, noise and restraint. In each case, activation of medullary adrenergic systems occurs either directly through visceral afferents or after processing of the stressful stimulus at a higher level. Thus, these circuits may be of importance under different conditions of stress and energy demand. Feeding responses are mediated by central alpha-2 adrenoceptors, whereas activation of central alpha-1 adrenoceptors suppresses food intake. As far as adrenergic systems are concerned, the lateral hypothalamic nuclei mainly have a hypophagic effect, mediated by central beta-2 adrenoceptors. Numerous experiments have been performed to pinpoint which structures in the brain are responsible for which cardiovascular responses. Although it is virtually impossible to study central adrenergic systems in man, an interesting observation comes from Australian investigators who sampled blood simultaneously from both jugular veins in patients with essential hypertension. The data show that there is a close relationship between central noradrenergic neuron activity and peripheral sympathetic activity (Ferrier et al. Peripheral Adrenergic Neurotransmission the information carried forward through preganglionic parasympathetic and sympathetic fibers is first relayed to postganglionic fibers and then via a neuroeffector junction to specific target organs. Finally, recent research has shown that several different mediators are co-transmitted at the same time (Macarthur et al. The most powerful mechanism of the body to regulate the effects of adrenergic stimulation, is by re-uptake 1. This is exemplified by the fact that inhibitors of this process can markedly potentiate the adrenergic symptoms while inhibitors of the uptake-2 mechanism have little effect. All these modulating factors make that the final response to an adrenergic stimulus is difficult to predict. Sympathetic Efferents the sympathetic nervous system can be viewed upon as a highly organized and differentiated system which transmits the final "order" that comes from the central nuclei, after they have integrated all afferent information, to the relevant effector organs. Neurophysiological studies in various species, including man, indicate that sympathetic neurons exhibit substantial differentiation with respect to their target cells and thus, their function (Janig and Habler, 2003). In other words: when a certain physiological function gets disturbed, the autonomic response, mediated through the sympathetic fibers, will be specifically directed towards the variable that needs to be regulated and will, in general, not affect other systems. Based on the final physiological responses, the system functionally consists of three main types of fibers: those that are involved in metabolic regulation (glucosensitive efferents), in the regulation of body temperature (thermosensitive efferents) and in the regulation of the cardiovascular system (barosensitive efferents). Glucosensitive Efferent Adrenergic Mechanisms Glucosensitive efferents are involved in the autonomic response to hypoglycemia and physical exercise or a state of negative energy balance. Finally, the autonomic system stimulates the liver to enhance gluconeogenesis and pancreatic cells to increase glucagon and reduce insulin output (Thorp and Schlaich, 2015; Watts and Donovan, 2010). It could explain also why diabetic patients have an ongoing increased sympathetic tone with all its metabolic and vascular consequences (Thorp and Schlaich, 2015). Thermal threats to the body include changes in both ambient temperature and in internal temperature due to . Again, most of our understanding of thermoregulation is based on experiments in animals but there are good reasons to believe that the situation is not much different in humans. Exposure of the body to cold activates two basic mechanisms, both of which are mediated by the sympathetic system: cutaneous vasoconstriction to prevent heat loss and thermogenesis (production of heat) by brown adipose tissue (Morrison, 2016). Constriction of skin vessels by adrenergic fibers is accompanied by visceral arterial and venous dilation to increase core blood flow. Stimulation of brown adipose tissue leads to an increase in energy expenditure due to enhanced oxidation of fatty acids and glucose. Although the brown fat was initially seen as a thermoregulatory organ in rodents and small mammals only, it is now known that this thermoregulatory effector system is also important in humans (van Marken Lichtenbelt et al. Except by cold, thermosensitive efferents are also activated by, for instance, emotional stimuli and hyperventilation. Basically, opposite reactions occur during heat stress, although the mechanism of cutaneous vasodilation is less well understood (Smith and Johnson, 2016). At first sight, one would think that the sweating response to heat is also adrenergic in nature. While this response certainly involves sympathetic fibers, these are cholinergic rather than adrenergic. Barosensitive Efferent Adrenergic Mechanisms By far the largest part of the sympathetic system is concerned with the control of the cardiovascular system.
Discount provera 5mg line
In such nodules menopause 7 keto dhea buy provera 10 mg free shipping, current studies focus on thermal ablation techniques, which are only available in a limited number of centers. Ultrasound-guided thermal ablation, whether with laser, radiofrequency, high-frequency ultrasound or microwaves, have been introduced for solid and cystic solitary, benign, nonfunctioning thyroid nodules. It may be repeated and significantly reduces pressure symptoms and cosmetic complaints. The fact that the spread of energy with thermal destruction can be better controlled, as opposed to chemical destruction by injection of ethanol, may favor these techniques in the long term. Controlled studies with surgery as the gold standard, and comparison of not only efficacy and side-effects but also quality of life and cost are awaited and may promote routine use. Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Clinical review: Nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: Systematic review including traditional pooling and Bayesian network meta-analysis. The advent of ultrasound-guided ablation techniques in nodular thyroid disease: Towards a patient-tailored approach. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: A systematic review. Ruling in or ruling out thyroid malignancy by molecular diagnostics of thyroid nodules. Thyroid and Irradiation Barbara Jarzab, Daria Handkiewicz-Junak, and Jolanta Krajewska, Maria Sklodowska-Curie Memorial Institute - Cancer Center, Gliwice, Poland r 2018 Elsevier Inc. Glossary Chernobyl accident the explosion of the nuclear power plant in Chernobyl, Ukraine, on 26 April 26, 1986. Differentiated thyroid carcinoma An endocrine malignant neoplasm arising from the follicular thyroid cell, which exhibits thyroid differentiation features. External irradiation the delivery of radiation to the human body by any external source of radiation. Effective radiation dose It is the tissue-weighted sum of the equivalent doses in all specified tissues and organs of the human body and represents the stochastic health risk (the probability of cancer induction and genetic effect) to the whole body. It is used to assess the potential for longterm effects that might be related to radiation exposure; it takes into account: (1) radiation dose, (2) the relative harm level of the radiation used (not all radiation is the same), (3) the sensitivities of each organ to radiation. Fukushima accident the accident of the nuclear power plant in Fukushima, Japan, on March 11, 2011. Internal irradiation the delivery of radiation to the body by ingested or inhaled radioactive isotopes. Radiation dose Amount of energy deposited in tissue as a result of an exposure to ionizing radiation. It is used to calculate the dose uptake in living tissue in radiation protection and radiology. Depending on the modality and severity of irradiation, the damage to the thyroid may cause cell death or may be less severe leading to specific genetic abnormalities. The thyroid is particularly vulnerable to the effect of ionizing radiation with the pediatric population being the most sensitive. Diagnostic and therapeutic medical radiation represents the largest source of man-made radiation exposure. However, the most striking increase in the incidence of thyroid cancer has occurred after Chernobyl nuclear plant accident, among children and adolescents who lived in the most contaminated areas of Belarus, the Russian Federation, and Ukraine. This article reviews the sources of thyroid radiation, radiation risk to the thyroid gland and the most relevant health consequences on the thyroid after external and internal irradiation. Source of Radiation Exposure People are exposed to natural radiation sources (background radiation) as well as human-made sources on a daily basis. Natural radiation comes from naturally occurring radioactive materials found in soil, water, and air. Beyond medical radiation nuclear accidents and nuclear bomb testing can be a source of high radiation dose in some regions. Both external radiation (therapeutic or accidental) and internal radiation (caused by therapeutic or accidental irradiation due to radioactive isotopes of iodine) have been associated with thyroid diseases in vitro as well as in vivo (Sinnott et al. Factors Affecting Sensitivity to Develop Radiation-Induced Thyroid Cancer Age and Sex Young age at the time of radiation exposure is a major risk factor with a strong inverse relationship between age at exposure and the risk of thyroid cancer development. According to a pooled analysis of 12 studies including 970 thyroid cancer patients exposed Change History: March 2018. Updated for Thyroid damage after radiation; age and sex; genetic predisposition; post-Chernobyl thyroid cancer, biology of post-Chernobyl thyroid cancer, glossary, and further reading and added the section for source of radiation exposure; Fukushima nuclear power plant accident; Prevention of thyroid cancer after internal contamination with radioactive iodine; Table 1. However, above the age of 15 the risk was not longer statistically significant (Sinnott et al. The data coming from a screening study of thyroid cancer prevalence among individuals exposed in utero to iodine isotopes suggested that such exposition may lead to an increased risk of thyroid cancer approximately 20 years later (Sinnott et al. Among other effects of in utero radiation exposure, observed after Chernobyl, were dose dependent and significant reductions in head and chest circumference without any significant effect of birth weight (Hatch et al. The risk of thyroid cancer after exposure to external radiation was believed to be two- to threefold higher in females than in males, but this gender effect was not confirmed in the recent pooled analysis of 12 studies, mentioned above, and was not found in contaminated children after the Chernobyl accident (Veiga et al. Thus, gender does not seem to influence the risk of developing radiation-induced thyroid cancer. It was reported that in some families several irradiated individuals developed thyroid cancer more often, that could be expected by chance (Iglesias et al. Patients who experience one radiation-related cancer are more likely to develop a second radiation-related cancer (Pacini, 2004). A higher risk was demonstrated in children irradiated for neuroblastoma and Hodgkin disease than in those, who underwent radiation treatment due to other reason (Rubino et al. However, studies available so far have not identified genetic determinants that modify individual predisposition to radiationinduced childhood thyroid cancer. Thyroid Damage After External Irradiation (Pacini, 2004) Irradiation to the head and neck for the treatment of distinct benign conditions. The relationship between irradiation and thyroid carcinoma for the first time was reported in 1950 (Duffy and Fitzgerald, 1950) and subsequently confirmed in numerous studies (Hanford et al. In 1970 in the United States up to 76% of children diagnosed with thyroid carcinoma had a history of previous radiation exposure (Pacini, 2004). Therefore, since 1970 external radiation for benign diseases has been totally abandoned in nearly all countries (Pacini, 2004). In Europe the incidence of radiation-induced thyroid carcinoma in children and adolescent was substantially lower: 10% in France and 7% in Italy (Pacini et al. Because of their smaller number of years at risk, the lifetime risk for adults is about one-fourth the risk for children. Little risk concerns persons after 20 years of age, and it is close to zero in persons after 40 years of age (Pacini, 2004). In a recent study, which included 12 large cohorts with 927 thyroid cancers after radiation exposure, the risk of thyroid cancer significantly increased after a mean dose to the thyroid during childhood as low as 0. In children exposed to a dose of 1 Gy to the thyroid, the relative risk of thyroid carcinoma ranges among series from 5. It was estimated that 88% of the thyroid cancers in this group of patients are attributable to radiation exposure (Sinnott et al. Thyroid and Irradiation 541 In most studies, the minimal latency period between the time of radiation exposure and the development of thyroid cancer ranges between 5 and 10 years. A shorter interval was noticed after the Chernobyl accident probably due to the large number of contaminated children among whom thyroid cancer was diagnosed earlier (Iglesias et al. The specific effect of diagnostic radiation exposure on thyroid cancer risk, previously believed to be controversial, has been unequivocally demonstrated by a meta-analysis of nine studies, just published. Considering a particular radiological examination both computed tomography and dental X-ray were associated with increased odds of thyroid cancer, 1. The thyroid damage is probably related to the distance between the thyroid gland and the radiation field. If this is far from the thyroid, as in the case of thoracic or abdominal radiation fields in children, the thyroid gland may receive minimal radiation doses of some 100 mGy, not enough to cause hypothyroidism but sufficient to trigger thyroid cancer (Pacini, 2004).
Diseases
- Ceramidase deficiency
- Sanfilippo syndrome
- Chromosome 9, duplication 9q21
- Kozlowski Rafinski Klicharska syndrome
- Acropigmentation of Dohi
- Abdominal defects
- Cardiomyopathic lentiginosis
- Thrombocytopenia Robin sequence
- Amnesia, drug-induced
- Hyper-reninism
Order 5 mg provera mastercard
In addition to single cells women's health clinic kilkenny order cheap provera on-line, small clusters are papillary-, trabecular-, microfollicular-, or syncytially patterned. Variably sized and shaped cells are spindle, polygonal, oncocytic, or bipolar with common binucleated and multinucleated cells. Differential diagnosis regards other tumors, such as anaplastic carcinoma and oncocytic neoplasms may be difficult based on cytology only. However, positivity for calcitonin and other neuroendocrine markers is diagnostic. Lymphoma Malignant lymphomas can arise in the thyroid gland as primary malignancies or involvement can be part of systemic disease. Hypercellular aspirates contain monotypic lymphoid cells in noncohesive sheets of single cells. Secondary tumors Tumors secondarily involving the thyroid gland are metastases from distant organs or direct extension of tumors from adjacent organs. The involvement can form three different patterns: (1) solitary large nodules, (2) multiple small discrete nodules and, (3) diffuse involvement. This subset of tumors was before classified as follicular variant of papillary carcinoma. The new entity should be managed as follicular adenomas, that is, lobectomy is indicated. Immunocytochemistry is the method to detect tissue antigens by utilizing specific antibodies and visualize antigen-antibody reaction by microscopically visible chromogen. Immunocytochemistry can be performed on cytological cell blocks or on cytological smears as well as on liquid-based specimens. Parathyroid lesions can be also distinguished from follicular lesions by immunocytochemistry. Poorly differentiated, anaplastic and secondary carcinoma can be distinguished from malignant lymphoma by specific antibodies. Quality Control and Assurance the specimen- should be monitored from the moment it arrives in the laboratory until the clinician receives the report. The Bethesda system for reporting thyroid cytopathology: Definitions, criteria and explanatory notes New York: Springer. American association of clinical endocrinologists, American college of endocrinology, and Associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-2016 update. Thyroid atypia of undetermined significance or follicular lesion of undetermined significance: An indispensable Bethesda 2010 diagnostic category or waste garbage Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. European thyroid association guidelines regarding thyroid nodule molecular fineneedle aspiration cytology diagnostics. The Bethesda system for reporting thyroid cytopathology: Proposed modifications and updates for the second edition from an international panel. Thyroid Carcinoma Rossella Elisei, University of Pisa, Pisa, Italy r 2018 Elsevier Inc. Some of these histotypes are really very rare and require to be managed in referral center by expert endocrinologists. The mean age at diagnosis is around 45 years but recently an increase of the mean age has been also observed with a plateau around 50 years (National-Cancer-Institute Surveillance, n. Children are very rarely affected and, although a more aggressive phenotype is observed at diagnosis, the prognosis is usually good with a very low rate of death (Sassolas et al. Other tumors can be present in the thyroid such as lymphomas and sarcomas or metastases of other human malignancy (Wood et al. They are all very rare but they must be taken into consideration for a differential diagnosis when an undifferentiated tumor is diagnosed. Several studies evaluated the consequences of the external radiation therapy on pediatric subjects suffering from benign diseases of the head and neck, such as tinea capitis, acne, chronic tonsillitis, skin angiomas, thymic hyperplasia. They showed a significantly higher incidence of thyroid nodules and then thyroid cancer compared to nonirradiated subjects (Brooks, 1973). Other risk factors are represented by the presence of a concomitant thyroid disease. Panel (A) Papillary thyroid cancer; panel (B) Follicular thyroid cancer; panel (C) Anaplastic thyroid cancer; panel (D) Medullary thyroid cancer. For these latter a longstanding goiter can be considered a risk factor since in many cases they are discovered in patients with a medical history of goiter (Molinaro et al. Sometimes, a lump in the laterocervical region(s) of the neck can be the first appearance, especially in children. In about 25% of cases, and in particular in young patients, it can metastasize through the lymphatic vessels to the lymph nodes, in particular to the cervical ones and the upper mediastinum. Although more rarely, the brain and the liver parenchyma can also be sites of metastases. For this reason, in 2009, the American thyroid association experts developed a new classification related to the risk of structural recurrence by defining three levels of risk: low, intermediate and high (Cooper et al. This classification is very important to plan the therapeutic and follow up strategies (Table 2). A similar concept has been developed for the radioiodine (131-I) treatment and, at variance with the past decades, nowadays a more personalized therapy is required thus confining the use of 131-I for the ablation of the postsurgical remnant to selected cases. Therefore, the initial treatment, which represents the most important step, should aim to reach a complete surgical removal of the tumor but avoiding iatrogenic complications which are more frequent when the surgery is more extended (Chen et al. A lot of controversial opinions and evidence have been reported about the need to add this procedure prophylactically. The lymph node dissection of the latero-cervical compartment(s), on the other hand, is still a matter of discussion. Sometimes echographic features of laterocervical lymph nodes are not clear enough to decide if a lymph node is metastatic or inflammatory. However, if this would not be the case and just a biopsy of the tumor can be the best surgical option, it is better to decline the surgical treatment and start with other therapeutic strategies (Haddad et al. Radioiodine Therapy Radioiodine (131-I) therapy can be used both for post surgical thyroid remnant ablation and for the treatment of distant metastases. Higher activities of 131-I must be reserved to advanced cases with incomplete surgical treatment or cases with already known distant metastases. No limits of cumulative dose have been identify and courses of high activities of 131-I can be repeated several times if renal and/or liver and/or lung functions are not yet compromised. From a clinical point of view it is indicated not to repeat 131-I courses in less than 6 months especially if lung lesions are present where the risk of pulmonary fibrosis can become a life treating event (Hebestreit et al. Although 131-I courses can be repeated many times without a real cumulative dose limitation, it is known that after a total of 600 mCi of 131-I it is very difficult to definitively cure the patient only with this type of treatment (Martins-Filho et al. It is evident that the follow up of both the cured and not cured patients is fundamental for the benefit of the patients. Patients with an excellent response to initial therapy have undetectable levels of these markers and their de novo detected positivity is expression of recurrence as well as the persistence of detectable levels is expression of persistent disease (Tuttle et al. Patients must be submitted to periodical controls whose schedule is very much dependent from the disease status. In cases with biochemical persistent disease the trend of increase of the serum marker becomes more relevant than the absolute value per se. A preferential use of some imaging techniques is usually recommended according to the anatomical district to be analyzed (Giraudet et al. In fact because of the lost of differentiation features they produce less or null amount of Tg. However, until recently, no effective therapeutic options were available for patients with any type of advanced thyroid cancer. Conclusions Thyroid carcinoma is represented by a variety of histotypes characterized by different biological behaviors.
Provera 5 mg with amex
Patient values should therefore be compared with those of age-matched normal subjects studied in the same conditions women's health boca raton buy provera 10 mg with visa. In such cases, alpha-blockers and non-dihydropyridine calcium channel blockers may be continued because they have little effect on renin release. In contrast, renin should be measured in patients prone to secondary hypertension. Such patients can be identified by their medical history, signs and symptoms, by the presence of hypokalemia, chronic kidney disease, abdominal bruit, or asymmetrical kidneys. Renin should also be measured in the small proportion of patients with severe untreated hypertension or in whom hypertension is resistant to at least a triple therapy including a diuretic, all administered at an optimal and maximally tolerated dosage. Renin-Dependent Forms of Hypertension (Table 2) Primary Hypertension About one in six patients with essential hypertension have high renin levels. The purpose and efficiency of screening for high renin hypertension in this setting is discussed above. The renin concentration is very high in patients with hyponatremic hypertensive syndrome, leading to hyperangiotensinemia with thirst, Table 1 Setting When to measure renin Usual presentation Key tests and findings 513 Table 2 Underlying disease Primary hypertension Benign Accelerated or malignant Non specific. Malignant hypertension may be primary or, as in 45% of cases, the consequence of an underlying renal or adrenal disease. About 80% of stenoses are due to atherosclerosis, usually in patients over the age of 50 years. Ten percent are due to fibromuscular dysplasia which is observed mostly in female patients. The captopril test, with concurrent determination of plasma renin levels, is predicted to induce a homeostatic increase in renin secretion from the stenotic kidney. Although the captopril test has been analyzed in 514 Hyperreninemia numerous patients at risk of having renovascular hypertension, a complete analysis is not possible because of multiple inconsistencies in patient selection, standards, test procedures, and cutoff points. In patients with fibromuscular dysplasia, defining the precise degree of stenosis may be challenging especially in patients with multifocal string-of-beads type of stenosis. Other Conditions Associated With Renal Ischemia A few conditions characterized by chronic or acute intra-renal ischemia may also induce renin-dependent hypertension. Segmental renal infarction may result from renal embolism, renal artery thrombosis or dissection, or in situ thrombosis in cases with coagulation disorders. It usually presents as an abrupt-onset malignant hypertension with hyponatremic hypertensive syndrome. The condition may revert spontaneously to normotension because the ischemic renal tissue, which releases large amounts of renin, may subsequently turn into a silent renal scar. Renal Tumors and Cysts Primary reninism is a very rare, purely renin-dependent form of hypertension. It may be caused by juxtaglomerular cell tumors, malignant kidney tumors (nephroblastoma, adenocarcinoma), epithelial or soft tissue tumors. In the 43 reported cases of juxtaglomerular cell tumors, hypertension was severe and associated with hypokalemia. Some large non-renin-secreting cysts and tumors may be found in cases of renin-dependent hypertension as the mechanism of renin stimulation is renal artery compression. Unilateral Non-Vascular Small Kidneys Pyelonephritic scarring associated with urinary tract infection and vesicoureteric reflux can cause childhood hypertension and progressive degradation of renal function. As mentioned above, rare cases of primary reninism may be due to extrarenal tumors. These conditions include dehydration, hemorrhage, diuretic use or pharmacological vasodilatation, cardiac failure, and reduced plasma volume due to hypoproteinemia in patients with nephrotic syndrome or kidney failure. Renin levels are related to survival in patients with cardiac failure, high levels being associated with a poor prognosis. The Hyperreninemia 515 prognostic value of atrial and brain natriuretic peptides is higher than that of renin, however, meaning that the latter is rarely used to assess prognosis. Recent advances in the field of molecular genetics have demonstrated that this disease is caused by four genetically distinct abnormalities that result from mutations in renal electrolyte transporters and channels. The prevalence of atherosclerotic renal artery stenosis in risk groups: A systematic literature review. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: A meta-analysis of randomized controlled trials. Inherited primary renal tubular hypokalemic alkalosis: A review of gitelman and Bartter syndromes. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: A systematic review and meta-analysis. Renal Vein Renin Measurement as a Diagnostic Tool Gian Paolo Rossi, University of Padua, Padua, Italy r 2018 Elsevier Inc. Experience has shown that it is clinically useful for diagnosing this condition as well as for identifying renin-producing tumors. Introduction the first to report an understanding of the phenomenon by which renal ischemia can raise blood pressure was Johan Loesch, a physician born in 1897 in a province of the Austro-Hungarian Empire, emigrated to the United States. In 1927 Loesch published in the Archives of Pathology a preliminary report of his studies on hypertension induced by renal ischemia, which he had performed in the Physiatric Institute in Morristown, New Jersey (Glodny and Glodny, 2006). After publishing few other papers out of the field of hypertension, Loesch moved to private practice and left research and there were no other scientific traces of his work. His seminal work was totally neglected by the scientific community and rapidly forgotten. In 1932 at the Academy of Medicine in Cleveland, Oh, Harry Goldblatt presented the results of several experiments that he and his colleagues had been performing during the previous 4 years by inducing renal ischemia in dogs (Goldblatt et al. Goldblatt and colleagues had invented a clip to constrict the renal artery and succeeded in inducing persistent elevation of blood pressure by clipping one renal artery. These results were eventually published in the Journal of Experimental Medicine in 1934. Peyton Rous, who was later awarded the Nobel Prize, on behalf of his co-Editor-in-Chief fully recognized the importance of the manuscript they had received and in the letter of acceptance wrote: It is a pleasure for us as editors to receive a paper of such large significance, so admirably written. This article is one of the most highly cited in the medical literature and stimulated more medical research than any single experiment in medical history. Nonetheless, strangely enough, Goldblatt did not receive the Nobel Prize in Medicine. Pathophysiologic Principles the induction of renal ischemia is followed within few minutes by renin release, both within the kidney and in the renal vein blood. This article is an update of Achille Cesare Pessina, Gian Paolo Rossi, Renal Vein Renin, In Encyclopedia of Endocrine Diseases, edited by Luciano Martini, Elsevier, New York, 2004, Pages 198-203. The fall of renal perfusion pressure, either induced experimentally or caused by a renal artery stenosis, determines an initial release of renin, which is followed by activation of renin synthesis in cell phenotypes, such as the vascular smooth cells in the afferent (preglomerular) arterioles wall, which, under physiological conditions, do not synthesize renin. The recruitment of these cells explains why both renin synthesis and renal renin content are markedly increased after induction of renal ischemia. Because of this time course the chances of detecting an activated renin angiotensin system and high plasma renin are, from the clinical standpoint, high when the patient is seen soon after a given renal artery obstructive lesion has become hemodynamically relevant, but wanes off afterwards. For these reasons, after the initial enthusiasms the measurement of plasma renin in peripheral plasma and in renal vein blood as a tool to diagnose renal ischemia and to predict the outcome of revascularization has lost ground, even though it still has an important place in the diagnostic work-up of at least two conditions, total renal artery occlusion and renin-producing tumors. The patient should be on a normal sodium intake, as verified by measuring 24-h urinary sodium excretion on the day of the test. Use of a long-acting calcium entry blocker, such as verapamil or a long-acting dihydropyridine compound, alone or combined with doxazosin, if necessary to achieve a decent blood pressure control before and during the test, can be allowed as these agents under chronic conditions negligibly affect renin secretion. Accordingly, it is not uncommon to draw diluted renal blood, for example, blood not draining solely from the kidney. Nonsimultaneous Sampling the use of a single catheter for the sampling of both renal veins can expose to the risk of detecting a factitious gradient between the kidneys or sites of sampling if the interventionist is not fast enough, particularly if an abrupt change in renin secretion occurs between the time of sampling on different sides/sites. Therefore, bilateral catheterization with simultaneous blood sampling is advisable unless the time elapsing between sampling from the two sides can be kept within 5 min. Furthermore, it is necessary to avoid any maneuver that can increase abruptly renin secretion, such as stressful stimuli, or administration of drugs (Table 1). With the newer chemiluminescent automated assays of immunoreactive active renin there are several advantages and less variability (Rossi et al. In fact, this assumption may not be verified if renin production is very high as in total renal artery occlusion or renin-producing tumours.
Effective 5mg provera
Different types of potassium channels have been implicated in functions such as salivary secretion pregnancy signs and symptoms buy generic provera 5mg line, bile and gastric acid secretion, protein digestion and absorption, insulin secretion, carbohydrate digestion and absorption, and taste transduction. Siani updated abstract, keywords, main text, references, further reading and deleted Table 3. Whereas extrarenal mechanisms that modulate potassium concentration inside and outside the cells are the first line of defense, the kidney is the final effector of longterm defense. Distribution of Potassium Between the Body Compartments Potassium homeostasis is finely regulated, so that wide variations in dietary potassium intake produce, in healthy individuals, only small changes in the total body potassium content and in the plasma potassium concentration. Therefore, loss of potassium through urine, stool, and sweat generally matches potassium intake, and potassium balance is maintained. Two major physiological mechanisms cooperate to achieve this end, namely, extrarenal mechanisms that control the tissue uptake and distribution of the electrolyte, and a renal tubular system that regulates potassium excretion as a function of acute and chronic changes in potassium intake (Gumz et al. Although only a small fraction of total body potassium in humans is in the extracellular space, minor changes in extracellular potassium concentration can be life-threatening (Pepin and Shields, 2012). Therefore, plasma potassium concentration must be maintained close to its setpoint. Cells act as an important storage pool for potassium, alternatively taking up excess potassium or releasing potassium, and operate to maintain its plasma concentration within the narrow limits allowed for physiologic function. Extrarenal Regulation of Potassium Balance Potassium can be actively transported into and can diffuse out of all cells. Extracellular potassium concentration is therefore regulated by cell membranes that act as effector organs, by buffering rapid changes in plasma potassium concentration, such as those occurring during the absorptive period. This system is highly effective, particularly in protecting against sudden changes in potassium concentration. Virtually every cell in the body uses this mechanism to maintain a high intracellular potassium concentration and a low extracellular potassium concentration (Palmer, 2015). There are several regulatory factors that control the distribution of potassium within body fluid compartments (Table 1). Both insulin release and epinephrine release increase Table 1 Determinants of transcellular potassium distribution Lower plasma potassium concentration Hormones Insulin b-Adrenergic agonists Aldosterone Metabolic alkalosis/respiratory alkalosis Increase plasma potassium concentration Cell destruction (hemolysis, crushing) Hormones a-Adrenergic agonists Glucagon Hyperchloremic acidosis Plasma hyperosmolality Drugs b2-Antagonists Angiotensin-converting enzyme inhibitors Digitalis Prostaglandin synthesis inhibitors Succinylcholine 554 Regulation of Potassium Homeostasis after meals, thus facilitating the movement of the ingested potassium to the intracellular space and preventing meal-related hyperkalemia. Hyperkalemia stimulates adrenal aldosterone secretion, which not only affects the renal excretion but also favors a shift of potassium to the intracellular space (Rabinowitz, 1996). Apart from hormonal factors, there are other extrarenal mechanisms that control the distribution of potassium within the body compartments. An acute rise in plasma osmolality causes an abrupt increase in plasma potassium, most likely due to the rapid exit of water from the cells, accompanied by an increased amount of potassium leaving the cell (Rastegar, 1990). Finally, several drugs (Table 1) could significantly affect extrarenal potassium homeostasis. Renal Regulation of Potassium Balance About 90% of dietary potassium is readily absorbed, mainly in the small intestine, mostly through passive mechanisms in response to electrochemical gradients and in proportion to the actual load (Agarwal et al. Circulating potassium concentrations remains relatively stable, even in the presence of wide variations in daily potassium intake, due the rapid cellular uptake of potassium entering the body after meals. This process, defined as extrarenal or internal potassium balance, has been discussed in the previous section and is under the control of the factors reported in Table 1. The long-term potassium homeostasis is regulated mainly by the kidney, which ultimately controls plasma potassium concentration as well as total body potassium. During conditions of neutral potassium balance, the daily intake of potassium is equal to the daily excretion rate, primarily in the urine. The remaining part of dietary potassium is eliminated in the feces and, to a lesser extent, in the sweat (Bailey et al. In healthy adults, the rate of potassium filtration by the glomerular capillaries is 756 mmol/day, considering a glomerular filtration rate of 180 L/day multiplied by a plasma potassium concentration of 4. Although there is a complex process of potassium reabsorption and secretion by the nephron from the proximal tubule through the loop of Henle, the bulk of filtered potassium load is reabsorbed in the proximal tubule and loop of Henle, so that o10% of the filtered load reaches the distal nephron. Beyond the early distal tubule, the tubule may modulate potassium delivery by either reabsorbing or secreting it, through both transcellular and paracellular pathways. Hence, urinary potassium excretion is ultimately regulated by changes in potassium secretion at the distal tubule and the collecting duct under the influence of a number of modulators (Giebisch and Wang, 2010; Table 2). Table 2 Determinants of renal potassium excretion Lower potassium excretion Decreased intracellular potassium concentration Decreased dietary intake Acidosis Unfavorable electrical or chemical profile Reduction of lumenal electronegativity Reduction of distal urine flow Reduction of distal sodium delivery or reabsorption Kidney function Decreased tubular filtration rate Tubular damage Diuretics Inhibitors of the apical sodium channel (amiloride, triamterene) Inhibitors of aldosterone Increase potassium excretion Increased intracellular potassium concentration Increased dietary intake Alkalosis Aldosterone Favorable electrical or chemical profile Increase in lumenal electronegativity Increase in distal urine flow Increase in distal sodium delivery Low lumenal chloride concentration Loop diuretics (furosemide) Regulation of Potassium Homeostasis 555 It should be considered that under most circumstances, the kidney primarily works to excrete (rather than to reabsorb) potassium to maintain potassium balance against variation in potassium intake from dietary or other sources. Under normal conditions, when ample potassium is consumed in the diet, there is an addition of potassium along the distal nephron; in the presence of a very high potassium intake, the distal nephron effects net secretion, whereas it effects net potassium reabsorption only in the presence of potassium restriction (Unwin et al. Cells in the connecting and collecting tubules respond to a number of signals by modulating their rate of potassium secretion over a wide range of values. First, the potassium load absorbed with a meal is the most important determinant of potassium secretion, because it is perceived as a stress upon the organism, which requires immediate response, through the involvement of multiple organs, including the gut, kidney, and the muscle and liver (Boyd-Shiwarski and Subramanya, 2017). Second, the feedback control of aldosterone secretion by the zona glomerulosa of the adrenal cortex is needed for regulation of potassium excretion. This in turn will favor the delivery of potassium into the renal tubule lumen down its concentration gradient, thereby stimulating potassium secretion. Glucocorticoids also affect potassium secretion primarily by increasing tubule fluid flow rate (Adam et al. Alkalosis induces greater potassium secretion at the distal tubule by moving potassium into the cells and acidosis moves potassium out of cells as hydrogen ions move in. The urine flow rate itself affects potassium secretion; with diuresis, increased amounts of fluid and salts move to the distal tubule, enhancing potassium excretion (Giebisch, 1998). Finally, a number of drugs can affect renal potassium handling by a variety of different mechanisms (Howes, 1995). For instance, loop diuretics, such as furosemide, in addition to increasing flow rate to the distal nephron, inhibit potassium reabsorption by the thick ascending limb of the loop of Henle. In contrast, the so-called potassium-sparing diuretics amiloride and triamterene block the apical sodium channel in the late distal tubule and collecting duct. The blockade of the apical sodium channel increases sodium excretion without affecting potassium secretion. More recently, it has been reported that renal potassium excretion has also a circadian rhythm independent of food. The circadian rhythm, which is regulated by a central clock in the brain, is transmitted to circadian clocks in the tubule cells responsible for variations in potassium excretion. As a result, potassium excretion is enhanced during the daylight phase and reduced during the night time phase (Gumz and Rabinowitz, 2013). Nutritional and Metabolic Aspects Potassium is largely present in many foods, particularly fruits, vegetables, whole grains, legumes, and nuts, typically in the form of organic (gluconate and citrate) salts, or, to a lower extent, of inorganic (bicarbonate and phosphate) salts. Estimates of the potassium content in different foods can be found in publicly available repositories. The total, nondiscretionary intake of potassium varies among different populations. In the International Collaborative Survey of Electrolyte Excretion and Blood Pressure, which analyzed 24 h sodium and potassium urinary excretion in over 10,000 individuals from 32 countries, the average potassium intake ranged from 25 mmol/day (American blacks in Goodman, Wisconsin, United States) to 87 mmol/day (Xingu Indians, Brazil) (Intersalt Cooperative Research Group, 1988). Potassium intake has been reported to be associated with several health outcomes, particularly cardiovascular endpoints. High dietary potassium intake may protect against developing hypertension and improve blood pressure control in patients with hypertension, while inadequate potassium intake may increase blood pressure (Aburto et al. Furthermore, there is consistent evidence from observational cohort studies that potassium intakes below 3500 mg (90 mmol)/ day are associated with a higher risk of stroke (Vinceti et al. As discussed earlier, major sustained increases in plasma potassium concentration are almost never caused by dietary changes, because the homeostatic mechanisms are highly efficient in healthy individuals. In response to a large oral potassium load, the excess potassium is partly stored in the large cellular compartment and partly excreted in the urine in a matter of a few hours. Thus, hyperkalemia may develop only in the presence of severe pathological conditions that impair the activity of these defense mechanisms. In fact, severe potassium depletion may occur when habitual potassium intake is o25 mmol/day (Young et al. One is the "protein-modified fast," which may be adopted by severely obese patients in the early phase of a weight-reducing program (Lin et al.
Discount provera 10mg free shipping
Indeed women's health center in grand rapids mi discount provera 5 mg with amex, limited data demonstrate that the combined therapy is associated with both the calcium lowering effect of cinacalcet and the skeletal advantage of bisphosphonates. Primary hyperparathyroidism: Review and recommendations on evaluation, diagnosis, and management. Medical management of primary hyperparathyroidism: Proceedings of the fourth international workshop on the management of asymptomatic primary hyperparathyroidism. Italian society of endocrinology consensus statement: Definition, evaluation, and management of patients with mild asymptomatic hyperparathyroidism. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. The surgical management of asymptomatic primary hyperparathyroidism: Proceedings of the fourth international workshop. Morphometric vertebral fractures in postmenopausal women with primary hyperparathyroidism. Glossary Bisphosphonates A group of drugs that inhibit osteoclastmediated bone resorption. Calcimimetics Allosteric stimulators of the calcium receptor that have been shown to lower serum parathyroid hormone and calcium concentrations in patients with primary hyperparathyroidism. Hungry bone syndrome A period of hypocalcemia that ensues after successful parathyroidectomy, during which calcium is rapidly deposited into bone and usual intake may not be sufficient to maintain serum calcium within the normal range. Positron emission tomography A clinical technique that is reported to successfully localize parathyroid cancer deposits. Preoperative localization Tests to localize the abnormal parathyroid gland prior to embarking on parathyroid exploration. Parathyroid carcinoma is an unusual neoplasm is characterized by a distinctive clinical profile that differs in several respects from that of hyperparathyroidism due to a benign parathyroid adenoma, which commonly presents with mild asymptomatic hypercalcemia (Shane and Bilezikian, 1982, Cohn et al. In this article, the clinical features, natural history, pathological features, diagnosis, and prognosis of parathyroid cancer are reviewed. Surgical approaches to parathyroid cancer are outlined as well as medical therapies of the hypercalcemia that accompanies recurrent or metastatic disease. Incidence In most series, parathyroid cancer accounts for less than 1% of patients with primary hyperparathyroidism (Brown et al. It is possible that parathyroid cancer is somewhat more common in Japan and perhaps also in Italy, accounting for 5% of patients operated on for primary hyperparathyroidism in series reported from these countries (Fujimoto and Obara, 1987; Obara and Fujimoto, 1991; Obara et al. Approximately 400 cases of parathyroid carcinoma were reported in the English literature between 1930 and 1999. In 1999, the National Cancer Data Base reported 286 cases of parathyroid carcinoma (Hundahl et al. In addition, an Australian retrospective case series reported that 3 parathyroid carcinoma cases were reported between 1958 and 1990, while 11 were reported between 2001 and 2010 (Brown et al. Possible reasons for this apparent rise in the incidence of parathyroid cancer include increased serum calcium screening, changing indications and increased surgical referrals for parathyroidectomy for asymptomatic hyperparathyroidism, and improvements in histopathological diagnosis. No clear pattern of predisposing factors has emerged in the cases that have been described. However, several clinical situations may predispose to the development of parathyroid cancer. Several cases of parathyroid carcinoma have been reported in patients with a history of neck irradiation (Christmas et al. Parathyroid cancer has also been reported within an adenoma or a hyperplastic parathyroid gland (Aldinger et al. Despite these associations, Shantz and Castleman, in an extensive review of 70 cases, found no evidence for malignant transformation of previously pathologic tissue (Schantz and Castleman, 1973). Parathyroid cancer has been reported in patients with familial hyperparathyroidism (Dinnen et al. In one such family, there was no evidence of antecedent hyperplasia in unaffected glands and chromosomal abnormalities commonly observed in other solid tumors were identified (a reciprocal translocation between chromosomes 3 and 4, trisomy 7, and a pericentric inversion in chromosome 9; Streeten et al. The authors concluded that no preoperative features distinguished hemodialysis patients with parathyroid carcinoma from those with parathyroid hyperplasia and that the clinical course may be more benign because of the tendency for renal insufficiency to lower serum calcium levels. Molecular Pathogenesis Oncogenes and tumor suppressor genes have been implicated in the development of parathyroid tumors. In the 21st century, there have been significant advances in the understanding of the molecular pathogenesis of parathyroid carcinoma. In one study, overexpression of cyclin D1 protein was found in 90% of parathyroid cancers (Vasef et al. However, it is uncertain whether overexpression of cyclin D1 protein is a causative or an associative phenomenon. Several other investigators have also reported that allelic deletions on chromosome 13 are more common in parathyroid cancer than in benign primary hyperparathyroidism. These data strongly support the presence of a tumor suppressor gene on the long arm of chromosome 13, critical for the development of parathyroid carcinoma. Another important cell cycle regulator, the p53 tumor suppressor gene, does not appear to be a major contributor to the pathogenesis of parathyroid carcinoma (Cryns et al. Parathyroid Cancer 141 Table 1 Parathyroid carcinoma and benign primary hyperparathyroidism: typical features Parathyroid carcinoma Primary hyperparathyroidism 3. Several locations have been identified for potentially important oncogenes or tumor suppressor genes that seem to be preferentially or exclusively found in parathyroid carcinomas as compared with adenomas. Tumor-specific gains or losses of chromosomal material suggest that oncogenes on chromosomes 1q, 5q, 9q, 16p, 19p, and Xq and tumor suppressor genes on chromosomes 1p, 3q, 4q, 13q, and 21q may be involved in the pathogenesis of parathyroid carcinoma. Thus, signs and symptoms of hypercalcemia dominate the clinical picture in addition to symptoms of hyperparathyroid bone disease and renal involvement (renal insufficiency, nephrolithiasis, or nephrocalcinosis). It is important to consider parathyroid cancer in the differential diagnosis of parathyroid hormone-dependent hypercalcemia, particularly in symptomatic patients, as often the diagnosis of parathyroid carcinoma is made in retrospect when hypercalcemia recurs due to local spread or distant metastasis of the tumor. The best outcomes for patients with parathyroid cancer occur when there has been complete resection of the tumor at the time of the first surgery (Shane and Bilezikian, 1982). Several features of the patient with primary hyperparathyroidism should suggest a malignant rather than a benign disease (Table 1). In contrast, there is no association of gender with parathyroid carcinoma and the ratio of affected women to men is 1:1 in most reported series. Patients with primary hyperparathyroidism typically present in their fifties or sixties, whereas the average patient with parathyroid carcinoma is in his or her forties, approximately 10 years younger. Thus, although it is reasonable to consider the possibility of parathyroid cancer when primary hyperparathyroidism is diagnosed in a man and or a younger individual, gender and age are of limited assistance in the setting of the individual patient. Signs and symptoms of hypercalcemia, including fatigue, weakness, weight loss, anorexia, nausea, vomiting, polyuria, and polydipsia are almost always present. Bone pain, fractures, and renal colic are much more common when primary hyperparathyroidism is due to a parathyroid cancer than an adenoma. Extremely high levels of parathyroid hormone are unusual in benign primary hyperparathyroidism, where circulating concentrations are commonly less than twice the upper limit of normal. In contrast, parathyroid hormone levels in patients with parathyroid cancer are generally much higher, ranging from 3 to 10 times above the upper limit of normal. On the contrary, non-functional parathyroid carcinomas do not present with hypercalcemia or hyperparathyroidism, and often present with a neck mass (Wilkins and Lewis, 2009). Serum alkaline phosphatase activity is often frankly elevated in patients with parathyroid carcinoma, whereas levels are usually in the normal or upper-normal range in patients with benign primary hyperparathyroidism (Silverberg et al. Finally, patients with parathyroid carcinoma may have elevated levels of a- and b-subunits of human chorionic gonadotropin, whereas patients with primary hyperparathyroidism do not (Rubin et al. This important clinical finding constitutes another striking difference between benign and malignant parathyroid disease. Recurrent laryngeal nerve palsy in a patient with primary hyperparathyroidism who has not had previous neck surgery suggests local spread of the tumor and is also very suggestive of parathyroid cancer.
Calcium D-Glucarate. Provera.
- Preventing breast, prostate, and colon cancer; and for detoxifying the body of carcinogens, toxins, and steroid hormones.
- Are there safety concerns?
- Are there any interactions with medications?
- Dosing considerations for Calcium D-glucarate.
- What is Calcium D-glucarate?
- How does Calcium D-glucarate work?
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96176

Order 10 mg provera otc
Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells breast cancer jordans order provera overnight. Brain natriuretic peptide increases in septic patients without severe sepsis or shock. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Uncoordinated regulation of atrial natriuretic factor and brain natriuretic peptide in lipopolysaccharide-treated rats. Evidence for load-dependant and loadindependant determinants of cardiac natriuretic peptide production. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Deconvolution analysis of cardiac natriuretic peptides during acute volume overload. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Gene expression, processing and secretion of natriuretic peptides: Physiologic and diagnostic implications. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Rationale and design of a randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy of B-type natriuretic peptide for the preservation of left ventricular function after anterior myocardial infarction. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Amino-terminal fragment of the prohormone brain-type natriuretic peptide in rheumatoid arthritis. The effect of atrial natriuretic peptide on plasma renin activity, plasma aldosterone, and urinary dopamine in man. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Distribution and characterization of immunoreactive porcine C-type natriuretic peptide. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. Elimination of up to 80% of human pancreatic adenocarcinomas in athymic mice by cardiac hormones. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: Impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. The pronatriodilatin gene is located on the distal short arm of human chromosome 1 and on mouse chromosome 4. Historically, mineralocorticoid was a primarily effector definition-a steroid hormone secreted from the adrenal cortex in response to sodium deficiency/volume depletion, to restore the status quo by increasing transepithelial retention of sodium in the kidney. Electrocortin was rapidly renamed aldosterone when its structure was established, in recognition of the unique aldehyde group at carbon 18 instead of the otherwise universal methyl group. It is more than 60 years since the isolation and characterization of aldosterone, and the hormone remains the central pillar of fluid and electrolyte homeostasis. In this complex context aldosterone still stands as the physiologic mineralocorticoid in terrestrial vertebrates, and as such a necessarily brief overview is in order. Aldosterone In evolutionary terms aldosterone is a uniquely terrestrial hormone, first found in lungfish-which, as their name implies, were the transition species from an obligate aqueous environment to the land: they still can be found in South Africa, South America, Australia (and Japanese pet shops). Circulating aldosterone levels are normally very low, to some extent reflecting the common sodium-replete diet in most countries. To some extent offsetting the B1000-fold difference in plasma concentrations of aldosterone and cortisol are the differences in plasma binding. One corollary of this is that the plasma half-time for aldosterone (B12min) is much lower than that of cortisol (B30 min). Another difference between the two is the shape of the nocturnal rise in steroid levels, much faster for aldosterone (and decaying faster) than for cortisol, the acuteness of the aldosterone response perhaps reflecting the volume shifts from systemic to pulmonary circulation upon recumbency. In humans there are family of 48 nuclear receptors (steroid/thyroid/retinoid/lipid-binding/orphan, the last without or with as yet undiscovered ligands); in mice there are 49. For several decades deoxycorticosterone was the recognized mineralocorticoid, as previously noted. Its physiologic role as a mineralocorticoid is minimal, for at least three reasons. Pathophysiologically, however, deoxycorticosterone may play a major mineralocorticoid role, in at least two circumstances. The ten-fold increase in sensitivity in the Kagawa bioassay when rats are adrenalectomized is in accordance with this scenario. There really can be no question that the metabolism of cortisol by the enzyme is so complete that it can not occupy the receptors. The intracellular concentrations of cortisol are (presumably, like plasma free levels) B100 times higher than those of aldosterone. In patch clamp studies on rabbit cardiomyocytes, cortisol normally acts as an aldosterone antagonist. Studies using Lagendorf preparations of rat hearts confirmed and extended this effect of cortisol. Under ischemia-reperfusion conditions, aldosterone increased the infarct size/area at risk, an effect blocked by spironolactone. In one cell type, however, it appears an unreserved mineralocorticoid receptor agonist. Normally these receptors cannot bind steroids, in that they are phosphorylated at serine 843 in the ligand binding site. This, incidentally, answers the conundrum of the different effects of aldosterone synthase and mineralocorticoid receptor gene deletion in mice. Conversely, in some studies it appears to have a very, very high affinity (Kd B1012) for aldosterone, with that for other corticosteroids to be determined: its (patho) physiologic significance remains to be established. Mineralocorticoid Excess Syndromes the first two cases of successful unilateral adrenalectomy for hypertension and hypokalemia were published in the Polish literature in 1953. Jerome Conn reported his findings in the following year: he had the twin advantages of aldosterone having been isolated and characterized and publishing in English. Secondary aldosteronism is a physiologic response to a low intravascular volume, as occurs in ascites. In patients able and willing to possibly undergo surgery lateralization is by bilateral adrenal venous sampling, to determine whether one or both glands are responsible. Patients who do not undergo adrenal venous sampling, and those who do not lateralize, are treated with a mineralocorticoid receptor antagonist, preferably at low dose, plus amiloride/triamterene and conventional antihypertensives as required: surprisingly, a lowered salt intake appears uncommon as a recommendation. If the patient lateralizes on adrenal venous sampling the recommended pathway is unilateral laparoscopic adrenalectomy, which essentially always cures the hyperaldosteronism, and restores the elevated blood pressure to normal in over half the patients, reducing it in the remainder.
Purchase 10mg provera with amex
The benefits of T appeared limited to men with baseline low T levels and treated for longer duration with T at relatively high doses women's health digestive problems order provera 10 mg on line, with possibly greater effectiveness of i. More recently, the Testosterone Trials investigated the efficacy and safety of T therapy in men with late-onset hypogonadism. Additional significant increases were seen on femoral and spine cortical and trabecular bone and estimated strength; the effect was greater on trabecular than on cortical bone, and greater on the spine than on the hip (Snyder et al. Therefore, current guidelines in male osteoporosis recommend that hypogonadal men with low T and low fracture risk may not need additional anti-osteoporotic medications, while men at increased fracture risk should be treated with approved fracture prevention drugs (Watts et al. Estrogens are essential for cortical and trabecular bone development in both genders; however, key elements of musculoskeletal sexual dimorphism are determined by androgen effects of periosteal bone formation and trabecular bone resorption. During midlife, men continue to consolidate their cortical and trabecular bone assets, likely independent of sex steroids. Although the clinical role of sex steroids in the diagnosis and treatment of osteoporosis in general is limited, there is growing interest and attention for bone loss and fracture risk in patients treated with hormone deprivation therapies for breast and prostate cancer. Further research is needed to investigate the cellular and molecular mechanisms by which sex steroid regulate bone metabolism, which could potentially lead to the development of new drugs to exploit their musculoskeletal benefits without adverse reproductive or cardiovascular effects. Bone health and bone-targeted therapies for nonmetastatic prostate cancer: A systematic review and meta-analysis. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham study. Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham heart study and applied to three geographically distinct cohorts. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: A randomized controlled trial. The role of activation functions 1 and 2 of estrogen receptor-alpha for the effects of estradiol and selective estrogen receptor modulators in male mice. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: A randomized, controlled trial. Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. Mineralization and bone resorption are regulated by the androgen receptor in male mice. Growth hormone and sex steroid effects on bone metabolism and bone mineral density in healthy aged women and men. Epidemiology of fractures in the United Kingdom 1988-2012: Variation with age, sex, geography, ethnicity and socioeconomic status. Genetically modified animal models as tools for studying bone and mineral metabolism. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. Androgens and skeletal muscle: Cellular and molecular action mechanisms underlying the anabolic actions. Clinical review: the benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: A systematic review and meta-analysis. Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. Gonadal steroiddependent effects on bone turnover and bone mineral density in men. Vertebral fractures and trabecular microstructure in men with prostate cancer on androgen deprivation therapy. Structural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapy. Comparison of bone mineral density and body proportions between women with complete androgen insensitivity syndrome and women with gonadal dysgenesis. Bone microarchitecture and estimated strength in 499 adult Danish women and men: A crosssectional, population-based high-resolution peripheral quantitative computed tomographic study on peak bone structure. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: Relative contributions of bone size and volumetric bone mineral density. Reproductive hormones and longitudinal change in bone mineral density and incident fracture risk in older men: the concord health and aging in men project. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: A randomized, placebo-controlled trial. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. Hormonal and biochemical determinants of trabecular microstructure at the ultradistal radius in women and men. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: Is a revision needed Relationship of volumetric bone density and structural parameters at different skeletal sites to sex steroid levels in women. Estrogen receptor alpha in osteocytes regulates trabecular bone formation in female mice. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: Validation of the free hormone hypothesis. Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Reproductive endocrinology: Functional effects of sex hormone-binding globulin variants. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Inactivation of estrogen receptor alpha in boneforming cells induces bone loss in female mice. Inactivation of the androgen receptor in bone-forming cells leads to trabecular bone loss in adult female mice. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice.
Buy discount provera on line
Iodized Water Water women's health healthy food purchase generic provera, like salt, is necessary for all humans, regardless of geographic or socioeconomic situation. As in salt, the addition of iodine to water can provide a constant physiologic dose. One of the simplest is to add a concentrated iodine solution to vessels containing drinking water. A program using this approach has been used in schools and homes in northern Thailand for several decades. Addition of iodine to running drinking water has been used in several countries. Another approach uses diffusers, commercially available polypropylene baskets containing iodide that is slowly released into a water source, such as wells. Finally, an innovative system in western China periodically drips potassium iodate into irrigation water. Molecular iodine (I2) has powerful bactericidal properties and is frequently used for water purification. Many communities needing water purification are also iodine deficient, so a system delivering molecular iodine can solve two problems at the same time. This is likely to be a declining source of iodine for two reasons; school children in particular are drinking less milk, and the increasingly widespread of organic milk which contains less iodine. Iodine Tablets and Solutions Tablets containing potassium iodide are occasionally used for iodine supplementation. They can be taken daily at 100 or 200 mg, weekly at 1 mg iodine, or at less frequent intervals up to several months, in correspondingly larger doses. The smaller doses may be used as supplements in special circumstances, such as pregnancy, and are also frequently incorporated into antenatal vitamin and mineral preparations. However the iodine content of prenatal multivitamins is very variable (Bath et al. Some countries, such as the United States, stockpile tablets containing 65 or 130 mg potassium iodide to block thyroidal uptake of radioactive iodine in case of a nuclear event, but these amounts are far too excessive for routine prophylaxis against iodine deficiency. Even remote health posts in developing countries frequently have iodine solutions for topical antisepsis. These can be appropriately diluted and used for iodine supplementation, again aiming at approximately 150 mg iodine/day. Such programs can be quite effective, but require responsible oversight to see that the correct dose is actually taken. Choice of Iodine Vehicle Iodized salt is by far the favored vehicle for most situations. It does not require individual contact, is technologically simple, is low in cost, and provides a constant daily supplement. In some countries, it requires a massive reorganization of the salt trade, so that an iodization step can be reasonably interpolated. For countries that import salt or have only a few large producers, introducing iodization is usually straightforward. Other countries may have many small salt farmers scattered over a large area who harvest the product simply and sell it locally. Some progress has been made by iodizing their product through cooperatives and other methods, but with only partial success. When iodized salt cannot be effectively introduced within a reasonable time, other methods need to be recruited. Oral iodized oil can be distributed rapidly through community health systems and provides a stopgap while awaiting salt iodization. Iodized water should be considered, depending on water sources and appropriate supervision. Iodized tablets and solutions are effective for individuals, but require careful administration. More recently bread has been used as iodine fortification, this is an attractive target as bread is widely consumed with similar intakes throughout the year. This approach has been introduced successfully in Australia, Denmark, and New Zealand (Seal et al. However follow on studies in New Zealand indicate that whilst effective it is still not as good as universal salt iodization (Edmonds et al. Many other vehicles have been occasionally used and may have a niche in certain locales. For each, one must ask whether its iodization will reach the right part of the population, especially the poor, women, and children, and in the right amounts. A possible danger is that several different forms of iodine supplementation may converge in an individual and produce iodine excess. Iodine Supplementation Strategies for Pregnancy and Lactation Women of child-bearing age are a key target for iodine supplementation programs. The correction of iodine deficiency in pregnancy results is in reduced infant mortality and reduced incidence of cretinism and is economically advantageous. As a result iodine deficiency can occur in pregnancy, despite iodine sufficiency in the general population. Universal salt iodization remains the best strategy for ensuring iodine sufficiency in pregnancy. It is also intriguing that both European and American Thyroid associations recommend 150 mcg daily for iodine supplements, even though the United States is more iodine sufficient than most of Europe. Patient education is key and planning prior to pregnancy may be essential as iodine supplementation may need to be undertaken well in advance of pregnancy for maximum benefit. Observational data suggests that consumption of iodized salt may need to be undertaken 2 years prior to pregnancy (Moleti et al. Iodine Deficiency Disorders Control Programs Background Programs for controlling iodine deficiency are organized by national governments or Health Ministries, as they relate to the public health of a country or region, and are best underpinned by government statute. This provides these programs with a legal Iodine Deficiency 519 Table 3 Guideline Summary of key guidelines for iodine supplementation in pregnancy Guidance To achieve a total of 250 mcg iodine ingestion daily in North America all women who are planning to be pregnant or are pregnant or breastfeeding should supplement their diet with a daily oral supplement containing 150 mcg of iodine Preconception and during pregnancy and breastfeeding, women should increase their daily iodine intake to 250 mcg. Iodine intake during pregnancy and breastfeeding should not exceed 500 mcg/day Women who are pregnant, lactating, or planning a pregnancy should ingest daily supplements containing 150 mcg iodine Women who are pregnant or breastfeeding take a daily oral iodine supplement so that the total daily intake is 250 mcg American Thyroid Association StagnaroGreen et al. Furthermore, governments have the ability to enforce mandatory legislation, ensuring their sustainability in the long term. However, national or regional governments need to be convinced of the need for controlling iodine deficiency, by individual experts and local or international "expert" organizations. Although governments take a lead role, the task of organizing successful programs requires a coordinated effort between many governmental and nongovernmental agencies for example, food and salt industries, education and health authorities. The involvement of the private sector has been a feature in many countries with successful programs. The public should be educated about the advantages of iodized salt and their use in preventing damage to their children, thereby creating a demand for iodized salt. The importance of establishing channels of communication with the government and public health education programs cannot be overestimated. A national multidisciplinary committee involving experts from medicine, nutrition, education, salt and food industries, media etc. The active involvement of the food and salt manufacturing industries is vital at this stage. If quality control measures for salt iodination and other well defined criteria are met, this would help in the elimination of iodine deficiency from regions that implement such programs (e) Monitoring-this phase of iodine deficiency control is often neglected, sometimes because of economic reasons. Monitoring requires logistical support and laboratory expertise, often not available in low income countries. The lack of monitoring has led to the failure of previously successful programs in the past. An important principle in monitoring is that it needs to be done both at the level of the salt manufacturer and the household and needs to be done reasonably frequently. The availability of iodine can change frequently and unpredictably with alterations in the physical, political, commercial, and cultural landscape. Natural disasters and civil unrest can disrupt salt plants and distribution patterns, a new government may overturn established patterns of health care and regulatory enforcement, changes in the management and structure of salt companies may profoundly affect the market for iodized salt, and understanding and concern about iodine nutrition and the importance of iodized salt may wax and wane with policy makers and consumers. Because at least some of these factors are likely to occur in any country, careful monitoring of iodine nutrition is essential to maintain effective optimal iodine intake in a population.
Order provera 10mg line
Although T4 can affect the expression of thyroid hormone-responsive genes breast cancer nails design generic 5mg provera with visa, the affinity of the thyroid hormone receptors for T4 is considerably lower compared to that for T3. Other Iodothyronines Progressive deiodination of T4 and T3 by the three known deiodinases gives rise to a number of different triiodothyronines and diiodothyronines. Of these, reverse T3 (3,30,50 -triiodothyronine) and 3,5-T2 (3,5-diiodothyronine) have also been shown to elicit T3-like effects. In the case of stimulation of mitochondrial activity by 3,5-T2, it was indeed shown to involve a direct interaction of this iodothyronine with mitochondrial enzymes. When administered at concentrations comparable to those of T4 and T3, these compounds show T3-like effects. These thyroid hormone analogs have the potential to rescue thyroid hormone signaling (Groeneweg et al. Although it cannot be ruled out that in certain circumstances cellular concentrations of these metabolites may reach levels high enough to elicit significant effects, they are generally considered to be of little or no physiological relevance based on the known low plasma concentrations. Mechanisms of Thyroid Hormone Action In the following sections, the mechanisms of the primary actions of thyroid hormone are presented. The first step in thyroid hormone action is actually transport of the thyroid hormone (T3 or T4) into the cell. We have learned much in the last several years about the classes of transporters responsible for intracellular thyroid hormone transport. We do not have any information about the mechanism of transport of the thyroid hormone from the cytoplasm into the nucleus. The in vitro effects correlate with increased activity of this enzyme in erythrocytes taken from hyperthyroid patients and decreased activity in those from hypothyroid patients. In vitro and in vivo studies also show that T3 and T4 stimulate glucose uptake in a variety of tissues, increase heart rate, and induce vasodilation, with effects appearing within minutes. These effects, however, are transient and it is not clear whether they are relevant under normal conditions. The molecular mechanism underlying the mostly membrane-associated rapid effects is also unclear. There is evidence of a direct interaction of T3 or T4 with membrane-bound proteins, thereby altering their properties. Based on a large body of data, an alternative hypothesis proposes that T3 and T4 affect membrane properties in a less specific way by becoming part of it. The physicochemical properties of the molecules are such that the lipophilic, iodinated phenolic part inserts readily in the outer leaflet of the lipid bilayer, whereas the hydrophilic amino and carboxyl groups prevent the molecule from crossing the membrane (it is for this reason that specific thyroid hormone transporters are required for cellular uptake). This partitioning in the membrane at normal levels of T3 and T4 reduces its fluidity, and this will affect the activity of membrane-associated enzymes. Such a mechanism may also account for the immediate effects of T3 and T4 on many mitochondrial membrane-bound enzymes, although this is a matter of debate. Irrespectively, the number of mitochondria, mitochondrial membrane density, and mitochondrial activity generally increase with increasing thyroid activity. However, the presence of high-affinity thyroid hormone-binding proteins in the inner mitochondrial membrane in hormone-responsive tissues, but not in refractory tissues such as testis and spleen, suggests a unique pathway of thyroid hormone action in mitochondria. As with T3, specific binding sites for T2 were identified in the inner membrane of rat liver mitochondria, however, the mechanism of T2 action on mitochondria is poorly understood (Davis et al. Changes in thyroid status also lead to alterations in the lipid composition and properties of membranes, most notably affecting the saturation of fatty-acyl chains of the phospholipids. These lipid alterations are thought to be related to the effects of thyroid hormone on the expression of lipid desaturases. Any subsequent effects on membrane-associated processes are therefore secondary to a nuclear-mediated action of the hormone. Receptor-Mediated Nuclear Action There are two known genes for thyroid hormone receptors. These factors may increase the capacity for tissue-specific fine-tuning of thyroid hormone effects. Some in vivo data support such a role, but the precise physiological relevance in different tissues is unknown. The rate of expression of a gene is basically determined by the ease with which these complexes are formed. Promoter activity and transcription rate are therefore terms that describe the frequency with which transcription of a gene is initiated following assembly of the complex. Gene transcription is primarily modulated by factors that either facilitate and stabilize the assembly of the transcription machinery or disrupt it. Addition of acetyl groups to histones relaxes Thyroid Hormone Action 457 the chromatin structure, whereas deacetylation closes it. These proteins are key factors in the regulation of gene expression because they can be recruited by transcription factors, including nuclear hormone receptors. Moreover, it was shown that T3 decreased expression of estrogen receptors in testis of gold fish (Marlatt et al. This not only relieves the repression but also further stimulates promoter activity. The essential classes of factors involved are depicted, but different proteins exist within each class, some of which show tissue-specific expression. This in turn opens up the local chromatin structure, allowing the assembly of an active transcription complex. The corepressor complex is replaced by coactivator proteins (CoAct) upon binding of T3 to its receptor. Stimulation of expression of this brain-specific regulatory factor may in part explain the critical role of thyroid hormone in brain development Myelin basic protein Major protein component of the insulating myelin sheaths surrounding axons Stimulation of expression of this receptor accounts for the potentiating effect of thyroid hormone on the adrenergic b1-Adrenergic receptor responsiveness of many tissues, such as liver, fat, skeletal muscle, and heart Thyroid hormone receptor b1. It uncouples mitochondrial respiration, thereby converting oxidative energy directly to heat Gene transcription inhibited by T3 Myosin heavy-chain b. Corepressors and coactivators appear to be equally involved in this mode of regulation but with exactly opposite effects compared to the positively regulated genes. Many genes are expected to be directly regulated by T3 based on the responsiveness of expression under various conditions. A selection of such genes are presented in Table 1 together with a short description of the function of each gene. Effects of Thyroid Hormone on Target Tissues the large number of different processes, factors, and mechanisms summarized in the previous sections provide an explanation for the variety of tissue-specific actions of thyroid hormone. As noted previously, many of the physiological effects of altered thyroid hormone levels are secondary to a limited number of direct 460 Thyroid Hormone Action actions of T3, and a large-scale analysis of gene expression indicates that the current list of genes that are directly regulated by T3 is far from complete. The effects of thyroid hormone on some of the major responsive tissues, based on both clinical and animal experimental data, are described in the following sections. The emphasis is on the effects for which the primary targets of thyroid hormone are known. Brain the development of the central nervous system is severely impaired in the absence of sufficient levels of thyroid hormone. The incomplete maturation of the brain is evidenced by neurological defects and mental retardation in humans. The timing of connections between axons, dendrites, and their targets in the developing brain is critical for the formation of the neuronal network. Diminished axonal growth and reduced dendritic arborization are evident in cerebral cortex, hippocampus, and cerebellum and in the visual and auditory cortex of hypothyroid rats. The expression of many genes is dependent on T3, but only a few have been shown to be directly regulated, such as myelin basic protein and brain-derived neurotropic factor. The action of T3 on the other genes may in part be permissive, which means that T3 is essential for the action of another factor. For example, the stimulation of expression of ornithine decarboxylase by growth hormone in the brain is absolutely dependent on thyroid hormone. This enzyme is essential in nucleic acid and protein synthesis, and thyroid hormone alone has no effect on its expression. Interestingly, in hypothyroidism all enzymes that are dependent on thyroid hormone eventually reach normal levels of expression.