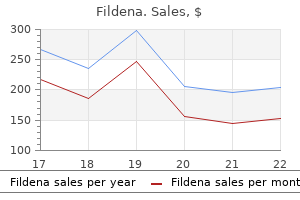
Purchase fildena once a day
Powders that are readily wetted by water when free of adsorbed contaminants are called hydrophilic erectile dysfunction protocol free copy buy generic fildena online. Surfactants are quite useful in the preparation of a suspension in reducing the interfacial tension between solid particles and a vehicle. As a result of the lowered interfacial tension, the advancing contact angle is lowered, air is displaced from the surface of particles, and wetting and deflocculation are promoted. However, at very high surfactant concentration, say, 15 times the critical micelle concentration, the surfactant produces extensive flocculation. Glycerin and similar the degree of flocculation is a more fundamental parameter than F because it relates the volume of flocculated sediment to that in a deflocculated system. The initial volume is Vo = 100 mL and the final volume of the sediment is Vu = 30 mL. Structured vehicles are pseudoplastic and plastic in nature; their rheologic properties are discussed in Chapter 19. As we shall see in a later section, it is frequently desirable that thixotropy be associated with these two types of flow. Caking diagram, showing the flocculation of a bismuth subnitrate suspension by means of the flocculating agent monobasic potassium phosphate. Apparently, glycerin flows in to the voids between the particles to displace the air and, during the mixing operation, coats and separates the material so that water can penetrate and wet the individual particles. The dispersion of particles of colloidal gums by alcohol, glycerin, and propylene glycol, allowing water to subsequently penetrate the interstices, is a well-known practice in pharmacy. To select suitable wetting agents that possess a welldeveloped ability to penetrate the powder mass, Hiestand7 used a narrow trough, several inches long and made of a hydrophobic material, such as Teflon, or coated with paraffin wax. At one end of the trough is placed the powder and at the other end the solution of the wetting agent. The rate of penetration of the latter in to the powder can then be observed directly. Controlled Flocculation Assuming that the powder is properly wetted and dispersed, we can now consider the various means by which controlled flocculation can be produced so as to prevent formation of a compact sediment that is difficult to redisperse. The topic, described in detail by Hiestand,7 is conveniently discussed in terms of the materials used to produce flocculation in suspensions, namely electrolytes, surfactants, and polymers. Electrolytes act as flocculating agents by reducing the electric barrier between the particles, as evidenced by a decrease in the zeta potential and the formation of a bridge between adjacent particles so as to link them together in a loosely arranged structure. If we disperse particles of bismuth subnitrate in water, we find that, based on electrophoretic mobility studies, they possess a large positive charge, or zeta potential. Because of the strong forces of repulsion between adjacent particles, the system is peptized or deflocculated. By preparing a series of bismuth subnitrate suspensions containing increasing concentrations of monobasic potassium phosphate, Haines and Martin10 were able to show a correlation between apparent zeta potential and sedimentation volume, caking, and flocculation. The addition of monobasic potassium phosphate to the suspended bismuth subnitrate particles causes the positive zeta potential to decrease owing to the adsorption of the negatively charged phosphate anion. Microscopic examination of the various suspensions shows that at a certain positive zeta potential, maximum flocculation occurs and will persist until the zeta potential has become sufficiently negative for deflocculation to occur once again. The onset of flocculation coincides with the maximum sedimentation volume determined. F remains reasonably constant while flocculation persists, and only when the zeta potential becomes sufficiently negative to effect repeptization does the sedimentation volume start to fall. These agents act as flocculating agents because part of the chain is adsorbed on the particle surface, with the remaining parts projecting out in to the dispersion medium. These workers10 also demonstrated a similar correlation when aluminum chloride was added to a suspension of sulfamerazine in water. In this system, the initial zeta potential of the sulfamerazine particles is negative and is progressively reduced by adsorption of the trivalent aluminum cation. When sufficient electrolyte is added, the zeta potential reaches zero and then increases in a positive direction. Colloidal and coarse dispersed particles can possess surface charges that depend on the pH of the system. An important property of the pH-dependent dispersions is the zero point of charge, that is, the pH at which the net surface charge is zero. The negative zeta potential of nitrofurantoin decreases considerably when the pH values of the suspension are charged from basic to acidic. The concentration necessary to achieve this effect would appear to be critical because these compounds can also act as wetting and deflocculating agents to achieve dispersion. Felmeister and others12 studied the influence of a xanthan gum (an anionic heteropolysaccharide) on the flocculation characteristics of sulfaguanidine, bismuth subcarbonate, and other drugs in suspension. Addition of xanthan gum resulted in increased sedimentation volume, presumably by a polymer-bridging phenomenon. Hiestand13 reviewed the control of floc structure in coarse suspensions by the addition of polymeric materials. Hydrophilic polymers also act as protective colloids, and particles coated in this manner are less prone to cake than are uncoated particles. These polymers exhibit pseudoplastic flow in solution, and this property serves to promote physical stability within the suspension. Gelatin, a polyelectrolytic polymer, exhibits flocculation that depends on the pH and ionic strength of the dispersion medium. Sodium sulfathiazole, precipitated from acid solution in the presence of gelatin, was shown by Blythe14 to be free-flowing in the dry state and not to cake when suspended. The coated material, precipitated from acid solution in the presence of gelatin, however, was found to carry a positive charge. This is due to gelatin being positively charged at the pH at which precipitation was carried out. It has been suggested8 that the improved properties result from the positively charged gelatin-coated particles being partially flocculated in suspension, presumably because the high negative charge has been replaced by a smaller, albeit positive, charge. Positively charged liposomes have been used as flocculating agents to prevent caking of negatively charged particles. Liposomes are vesicles of phospholipids having no toxicity and that can be prepared in various particle sizes. Flocculation in Structured Vehicles Although the controlled flocculation approach is capable of fulfilling the desired physical chemical requisites of a pharmaceutical suspension, the product can look unsightly if F, the sedimentation volume, is not close or equal to 1. Consequently, in practice, a suspending agent is frequently added to retard sedimentation of the flocs. Such agents as carboxymethylcellulose, Carbopol 934, Veegum, tragacanth, and bentonite have been employed, either alone or in combination. This can lead to incompatibilities, depending on the initial particle charge and the charge carried by the flocculating agent and the suspending agent. For example, suppose we prepare a dispersion of positively charged particles that is then flocculated by the addition of the correct concentration of an anionic electrolyte such as monobasic potassium phosphate. We can improve the physical stability of this system by adding a minimal amount of one of the hydrocolloids just mentioned. No physical incompatibility will be observed because the majority of hydrophilic colloids are themselves negatively charged and are thus compatible with anionic flocculating agents. If, however, we flocculate a suspension of negatively charged particles with a cationic electrolyte (aluminum chloride), the subsequent addition of a hydrocolloid may result in an incompatible product, as evidenced by the formation of an unsightly stringy mass that has little or no suspending action and itself settles rapidly. Under these circumstances, it becomes necessary to use a protective colloid to change the sign on the particle from negative to positive. This is achieved by the adsorption on to the particle surface of a fatty acid amine (which has been checked to ensure its nontoxicity) or a material such as gelatin, which is positively charged below its isoelectric point. We are then able to use an anionic electrolyte to produce flocs that are compatible with the negatively charged suspending agent. Rheologic flow curves of various suspending agents analyzed in a modified Stormer viscometer. Rheologic Considerations the principles of rheology can be applied to a study of the following factors: the viscosity of a suspension as it affects the settling of dispersed particles, the change in flow properties of the suspension when the container is shaken and when the product is poured from the bottle, and the spreading qualities of the lotion when it is applied to an affected area.
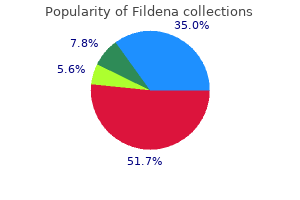
Purchase fildena 25 mg
On rotary tablet machines diabetic erectile dysfunction icd 9 code discount fildena 50 mg overnight delivery, multiple punches and dies are located around the outside of a circular die table and the compression process described above occurs as they are rotated under circular compression rolls that force the upper and lower punches together and punch guides pull them apart with precise timing. The entire process described above can occur on a production tablet press in less than 100 milliseconds. The compression process has been studied in detail by a number of investigators and a variety of equations to describe the relationship between compression pressure and tablet density have been developed. The yield strength of a material is a measure of its ability to permanently or plastically deform as discussed in the previous section on mechanical properties. A highyield pressure indicates that a material is hard; a low-yield pressure indicates a soft material. The true density of a material is its density in the absence of pores, meaning that the material contains absolutely no void space between particles. The reader is directed to Chapter 18 on Micromeritics for further discussion of density and methods of measurement. Virtually all pharmaceutical tablets have some porous structure though, and typical values for relative density are in the range of 0. The K is the slope of the Heckel equation and is a measure of the plasticity of the material. A greater slope indicates that the material has greater plasticity and is more easily permanently deformed. The different terminal slopes indicate that these three materials have significantly different deformation properties. The initial nonlinear region of the plot is the region in which the Heckel equation does not apply and reflects the initial stage of consolidation where significant particle rearrangement is occurring. The entericcoating material is insoluble in the acidic fluid of the stomach but dissolves in the relatively neutral pH of the intestine. Enteric coating is therefore a way of protecting acid labile drugs from being exposed to the harsh acidic stomach media that can degrade some active ingredients. Controlled release polymers may also be applied to dosage forms to control the rate at which drug enters the intestinal tract and is absorbed. Capsules are solid dosage forms in which the medicinal agent and excipients are enclosed in a small shell of gelatin. Two-piece capsules consist of a capsule body in to which the formulated material can be filled and the slightly larger diameter cap that slips over the body to seal the capsule. Soft gelatin capsules may be filled with liquids or semisolid ingredients, whereas the two-piece capsules are very often filled with dried powders. Recent advancements in two-piece capsule technology now allow for liquid and semisolid fills. Capsule machine equipment is designed to move the formulation in to the capsule body followed by positioning and closing the cap to produce the final product. One of the main advantages of capsule formulations is that they do not have to undergo the compaction process as tablets do. Industrial capsule machines are capable of fast manufacturing speeds though capsule machines do not currently reach the dosage form output of tablet machines. While the previous sections have covered some of the details of current pharmaceutical manufacturing processes which are done in batch mode, the future of pharmaceutical manufacturing is moving toward continuous processing. Continuous processing often combines one or more pharmaceutical processes utilizing equipment designed to allow for continuous input of starting materials, material processing, and continuous exit of final processed material. A continuous process inherently provides an opportunity for improved quality and consistency as it involves processing a much smaller quantity of material at any one time. For example, in a typical batch wet granulation unit operation involving a batch size of 300 Kg, the entire quantity is processed simultaneously. For a continuous operation with reasonable material throughput, the quantity of material being processed at any given time may be only about 400 g. Heckel and other equations have been used to interpret and predict the compaction properties of pharmaceutical materials and formulations. Because of the critical importance of the compaction process in forming tablet dosage forms, a great deal of research has revolved around understanding and modeling this process. The reader is directed to other comprehensive discussions of powder compaction for further information. The formulation must have the necessary properties to ensure consistent powder flow properties that allow it to move through the manufacturing equipment, including movement from any intermediate storage containers to the tablet press hopper and feeder system that directs the powder down in to the tablet die. Following compression, the tablet must have acceptable aesthetic properties such as a smooth, elegant appearance without cracks or chips. It must also be robust enough to handle any remaining processing that is required such as coating and packaging, yet consistently meet all the performance characteristics that ensure satisfactory performance such as disintegration and dissolution. The sugarcoating process seals and protects the tablet dosage form and, with the incorporation of color, adds a distinctive look and taste to the tablet. Many tablets are film-coated today as it is a more cost-efficient process than sugarcoating. A thin polymeric layer with a color added is sprayed on to the surface of the tablets to provide a distinctive appearance. Process and statistical modeling can be used to develop control strategies consistent with the Quality by design (Qbd) initiative. In many instances, it is possible to make large-scale lots with the same equipment used during small-scale development activities by running the process longer. Finally, as the unit operations are integrated in to a continuous process, there may be considerable material and resource savings. Among the commonly used unit operations in solid oral dosage form development, milling, roller compaction (dry granulation), compression (tableting), encapsulation, and packaging are inherently continuous processes. Over the last decade, a concerted effort from industry and equipment manufacturers has resulted in significant progress being done to make these unit operations continuous. As an example, for wet granulation, a modified twin screw extruder and other similar equipment have been designed for use. Several designs of low and high shear continuous dry blending equipment are also being marketed. With active research and development activities underway, significant advancement is expected to occur in the equipment engineering as well as overall process integration to make this approach for solid oral dosage from development a success in the next 5 to 10 years. Following the manufacture of tablets or capsules, final finishing of the dosage forms takes place. Two-piece capsules, for example, may be polished to remove small amounts of powder that may adhere to the outside of the capsules during filling. On a large scale, many capsule and tablet machines are affixed to a cleaning vacuum that removes extraneous material from the tablets or capsules as they leave the machine. Following manufacture, the tablets and capsules may be stored in bulk containers until they are packaged. The final dosage form packaging plays a critical part in ensuring and maintaining product quality. Drugs that are adversely affected by light will be packaged in light-resistant containers, whereas moisturesensitive drugs may be packaged with a desiccant to ensure that the dosage forms are not exposed to high moisture levels that could cause physical or chemical degradation. Properly stored dosage forms will remain stable and effective throughout the entire labeled shelf life of the product. Pharmaceutical ingredients which bond well together are capable of forming tablets with high strength. An old rule of thumb for tablet hardness was that the tablet should break with a sharp snap when squeezed between the fingers and thumb. Commercially available tablet hardness testers are available to provide quantitative data on tablet hardness. Tablet hardness is the force necessary to cause a tablet to fracture when compressed between two rigid platens.
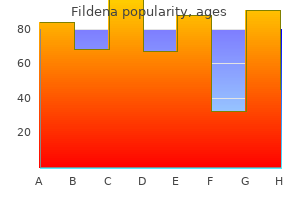
Purchase fildena 150 mg on line
Steri-strips are applied perpendicular to the wound when nonabsorbable 4-0 synthetic closure of the subcuticular skin is accomplished popular erectile dysfunction drugs purchase fildena 25mg. Suction catheters should remain in place Chapter 19 Modified Radical Mastectomy and Total (Simple) Mastectomy 299 for approximately 5 to 7 days or until drainage becomes serous and less than 20 to 25 cc per catheter over a 24-hour interval. Vigorous shoulder activity together with arm range of motion exercises should be delayed until drainage catheters are removed and should not be initiated aggressively. Range of motion exercises may be initiated the day following drainage removal but should be done progressively to avoid elevation of the adherent flaps on the chest wall. With protracted serous or serosanguinous drainage, continued suction may be utilized via the lateral-most (dependent) catheter. Long-term catheter use requires the patient to be instructed in hygienic care of the catheter and skin wound, as well as frequent dressing changes. These investigators suggest that improvement in measurements of flexion, abduction, and adduction motion of the shoulder joint and the functional scores were better following home exercise program management after removal of drains. Thus, early-onset rehabilitation following modified radical mastectomy will provide improvement in shoulder mobility and functional capacity without initiating adverse effects in the postoperative period. This study confirmed a trend toward enhancement of overall survival following the radical mastectomy, which was superior to the modified technique after 5 years (84% vs. Additional prospective studies confirming the similarity of outcomes with the modified technique were reported by Staunton et al. Given the excellent outcomes for control of disease, these authors concluded that the therapeutic outcomes with minimal morbidity allow the modified radical mastectomy to be considered the superior choice in treating the patient with primary operable cancer of the breast. Total (Simple) Mastectomy the clinical and surgical application of the term "total mastectomy" is synonymous with simple mastectomy. This procedure represents a modification of the modified radical mastectomy, in that preservation of the pectoralis muscles is ensured, but the axillary lymph nodes are dissected only at the level of the axillary tail of Spence nodes (level I). Thus, total mastectomy advocated the use of regional node dissection to treat local disease that appears clinically and anatomically confined to the breast by imaging techniques. In the evaluation of 2,243 patients with a mean follow-up of 11 years, results confirm no statistical differences in overall survival benefits between the two techniques. Recurrence rates appear to be proportional to tumor grade, and prophylactic irradiation was proposed to treat patients at high risk for recurrence. In the B-04 trial of the late 1970s, 1,655 patients with an average follow-up of 11 years were reported by Fisher et al. This study compared total mastectomy with and without axillary radiation with radical mastectomy. Final analysis confirmed no differences in disease-free survival rates between the groups with clinically negative nodes; there were no differences in disease-free overall survival rates between total mastectomy with irradiation and radical mastectomy for patients with positive lymph nodes. This number decreases in the later years, because there are fewer patients with relevant trial times. Disease-free survival through 10 years (A), during the first 5 years (B), and during the second 5 years for patients free of disease at the end of the fifth year (C). There were no significant differences among the three groups of patients with clinically negative nodes or between the two groups with clinically positive nodes. For node-negative patients, there were no significant differences in distant disease occurring as a first treatment failure among the three groups, whereas local and regional disease was best controlled in the group receiving radiation. For node-positive patients, there was no significant difference in distant or local and regional disease between the two groups. Diseasefree survival through 10 years (A), during the first 5 years (B), and during the second 5 years for patients free of distant disease at the end of the fifth year (C). Disease-free survival through 10 years (A), during the first 5 years (B), and during the second 5 years for patients alive at the end of the fifth year (C). There were no significant differences among the three groups of patients with clinically negative nodes or between the two groups with positive nodes. Moreover, the trial confirmed results following 5 years accurately predict the outcomes at 10 years. Prophylactic Total Mastectomy in the High-Risk Patient the sequencing of the human genome with advances in molecular biology and nanotechnology has enhanced the ability of the physician-scientist to predict risk for breast cancer. Guidelines proposed by the position statement of the Society of Surgical Oncology published in 1995 are included in Table 19. Patients undergoing prophylactic mastectomy had no incidence of breast cancer, whereas the surveillance group developed cancer at a rate that was comparable to that of other patients with the same genetic mutation. Despite its short-term mean followup, the results are positive for the prophylactic technique but should be interpreted in light of the short duration of this follow-up. Previous retrospective analyses of the highrisk patient were conducted by Mayo Clinic surgeons, Hartmann et al. Of significant importance are the technique utilized and the differentiation of the subcutaneous (incomplete central) mastectomy with that of the total mastectomy technique in which all tissues at risk are removed. Subcutaneous mastectomy has afforded excellent cosmetic results, but it should not be considered an oncologic procedure. This paper is important in that there was a reduction in risk of contralateral breast cancer in these patients by approximately 90% following the preventative procedure. Preservation of the pectoralis major muscle enhances volume and contour of the chest-wall deformity. Preservation of the thoracodorsal neurovascular bundle and long thoracic nerve enhances shoulder and arm functionality. Results of modified radical mastectomy are equivalent to those of the Halsted radical procedure. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June 1889 to January 1894. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. Management of early cancer of the breast: report on an international multicentre trial supported by the Cancer Research Campaign. The role of radiotherapy following total mastectomy for patients with early breast cancer. Role of radiotherapy following total mastectomy in patients with early breast cancer. A Danish randomized trial comparing breast conservation with mastectomy in mammary carcinoma. The primary surgical treatment of carcinoma of the breast: a changing trend toward modified radical mastectomy. Conservative treatment versus mastectomy in T1 or small T2 breast cancer: a randomized clinical trial. Comparison of Halsted mastectomy with quadrantectomy, axillary dissection, and radiotherapy in early breast cancer: long-term results. Significance of location and number of axillary metastases in carcinoma of the breast. The prognosis of carcinoma of the breast in relation to the type of operation performed. A review of 146 cases of carcinoma of the breast operated on between 1930 and 1943. The effectiveness of early rehabilitation in patients with modified radical mastectomy. A randomized prospective trial of radical (Halsted) mastectomy versus modified radical mastectomy in 311 breast cancer patients. Does radical mastectomy still have a place in the treatment of primary operable breast cancer A 25-year prospective study of modified radical mastectomy (Patey) in 192 patients.
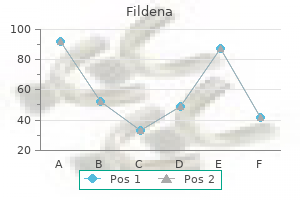
Discount fildena 50 mg with visa
One aspect of this poor understanding is a lack of availability of potential stabilizers for use as excipients in biopharmaceutical and vaccine formulations impotence from alcohol buy fildena 100mg. A practical problem with biopharmaceuticals is their manufacture at a scale sufficient for use in large populations. For example, in the case of monoclonal antibodies it appears that there is insufficient manufacturing capability if a significant number of such proteins currently in clinical trials come to fruition as marketed pharmaceuticals. The manufacture of live agents such as viruses has always proven to be challenging at an industrial scale. Thus, the development of new technologies to aid in the high-level manufacture of biopharmaceuticals is an important goal of modern biotechnology. As biotechnology-based products begin to go off patent, the possibility and then the reality of less expensive versions of these drugs and vaccines has become apparent. Both the terms "follow-on biologics" and "biosimilars" have been applied to such drugs. The major area of controversy with follow-on recombinant proteins has been the extent to which extensive clinical trials are necessary to ensure their safety and efficacy. In particular, are physical and chemical comparisons of biosimilars to the original innovator drugs sufficient to ensure these critical properties Scientists themselves expressed concerns that it might be difficult to predict the result of the alteration and insertion of new 559 genes in to novel cellular environments. At the public level, this went so far as to imagine the creation of genetically altered organisms with unique pathological characteristics and plants, which might spread deleterious genes in to nontarget plants. All of the proceeding can and have occurred, but so far without any significant disasters. Initially, we perhaps forgot that plant and animal breeders have been doing the same thing for hundreds if not thousands of years with essentially positive results. While the potential for problems and negative public perceptions remain real, the success of modern biopharmaceuticals and vaccines has maintained forward momentum in the use of these technologies. Several recent problems, however, illustrate continued negative perceptions in a minority of the population. For example, the use of stem cells derived from embryos to treat various diseases has raised much controversy due to the source of the cells. Claims that the measles vaccine or the compound thimerosal (a mercury-containing preservative) in certain vaccines causes autism in children are completely unsubstantiated by scientific evidence but have resulted in significant public concern, nevertheless. Russell, Condensed Protocols from Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2006. Golemis, Basic Methods in Protein Purification and Analysis: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2008. Sofer, Handbook of Process Chromatography: Development, Manufacturing, Validation and Economics, 2nd Ed. Gosling, Immunoassays: A Practical Approach, Oxford University Press, New York, 2000. Bloomfield, Laser Light Scattering in Biochemistry, Science and Behavior Books, Palo Al to , 1992. Pecora, Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics, John Wiley & Sons, Inc. Bromberg, Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology, Garland Science, New York, 2003. In dramatic form, these three films well illustrate the promise and problems of biotechnology. Recommended Reading It is recommended that the interested student view three excellent films, which provide a nice overview of the history of biotechnology. Describe preformulation development activities and their importance in developing a drug product. Understand the important physical, chemical, and mechanical properties of pharmaceutical materials and their relevance in formulation development. Understand the importance and role of oral dosage form performance tests in ensuring product quality and performance. For the drug to exert its biological effect, it must be released from the dosage form, permeate through biological membranes, and reach the site of action. The goal of this chapter is to provide an introduction to how these topics are integrated in to dosage form design, product development, and manufacturing activities. The focus of this chapter is on oral drug delivery, and in particular solid dosage forms. However, many of the basic principles apply to the design and manufacture of all types of pharmaceutical dosage forms. The pharmaceutical industry is, after all, a drug product industry, not a drug industry. The oral cavity provides the first contact with biological fluids where mastication and mix- ing with saliva takes place and digestion begins. As ingested components are swallowed, they move through the esophagus in to the stomach. It processes food in to chyme with vigorous contractions that mix the ingested contents with gastric secretions that continue digestion. It also regulates the input of these liquefied components in to the intestinal tract and serves as a major site of chemical and enzymatic breakdown. As stomach contents empty, the chyme enters the small intestine where the absorption of a majority of drugs and nutrients takes place. Absorption of drugs and nutrients can occur from each section of the small intestine and colon. The small intestine is partitioned in to three sections: the duodenum, the jejunum, and the ileum. For most drugs, the duodenum and the proximal jejunum are the best sites of absorption as they have the highest absorptive surface area and often the highest concentration of dissolved drug is achieved in the lumen of this region. Small intestinal absorption is now understood to be dramatically affected by regional differences in the distribution of transporters, enzymes, and greater detail is provided on these aspects in Chapter 12 on Biopharmaceutics. Significant drug absorption from the colon may also occur although the absorptive surface area is substantially less than that of the small intestine. Drug absorption may also occur from the oral cavity3-5 or, rarely, the stomach depending upon the drug and dosage form properties, which must be conducive to absorption from these sites. Many orally administered drugs enter systemic circulation via a passive diffusion process through the small intestine, although paracellular and transport-mediated absorption also occurs and our understanding of these absorption mechanisms continuous to grow. It also facilitates the identification of appropriate oral dosage forms and strategies to consider that provide opportunities to overcome physicochemical limitations. A detailed analysis of the transport and absorption of drugs is described earlier in this book and the student is directed there for a detailed discussion of the various aspects to relate to intestinal absorption. Note that C1 and C2 are the concentrations of drug inside the membrane, but since these are very rarely known or measured, a distribution coefficient, K, is typically introduced in to this equation to transform the concentrations to the respective aqueous concentrations on the bulk aqueous donor, Cd, and receiver, Cr, sides. The distribution coefficient reflects the tendency of the drug to partition in to the membrane and is the ratio of the drug concentration in the membrane (C1, C2) to that in the aqueous phase immediately adjacent to the membrane (Cd, Cr). A lipophilic drug would have a distribution coefficient greater than 1 since biological membranes tend to be lipophilic. With the presence of solid drug in the intestine, the concentration of drug dissolved in the intestinal tract, Cd, may approach or equal its aqueous solubility if dissolution of drug from the dosage form is sufficiently rapid that it is not rate limiting. Finally, a drug is considered highly permeable when the extent of absorption in humans is determined to be >90% of an administered dose, based on massbalance or in comparison to an intravenous reference dose. These include in vivo intestinal perfusion studies in humans, in vivo or in situ intestinal perfusion studies in animals, in vitro permeation experiments with excised human or animal intestinal tissue, or in vitro permeation experiments across epithelial cell monolayers. Characterizing the properties of the drug, also known as preformulation characterization, provides the information necessary to classify drugs and identify suitable dosage forms to address drug delivery issues. Back in an era when local pharmacies offered a delivery service, drug delivery was described as "a boy on a bicycle. An oral solid dosage form of chloroquine phosphate should conform to the following:19 Utilize standard excipients. Because of the challenges associated with drug discovery and development, the opportunity to identify and develop a safe and effective product benefits greatly from the integration of pharmacology, chemistry, toxicology, metabolism, clinical research, thorough physicochemical characterization and, very importantly, dosage form development.
Order fildena 50 mg without prescription
Dicloxacillin is safe for the suckling infant erectile dysfunction medication for diabetes order discount fildena, but if another antibiotic is chosen because of an unusual or resistant bacteria, safety for the infant should be addressed since the medication and/or metabolites are routinely transmitted via the breast milk. Antibiotics are prescribed as mentioned earlier, and the patient returns for repeat ultrasound and reaspiration every other day until no fluid remains in the cavity. For larger abscesses, a small percutaneous closed suction drain can be placed as an alternative approach to serial aspiration. Any of the following constitutes failure of nonoperative treatment and is an indication for surgical drainage: worsening systemic illness despite aspiration and antibiotics, abscess cavity does not progressively diminish in size, or local progression evidenced by skin necrosis or other changes. Other indications for initial operative drainage are very large abscess size, complicated and extensive pattern of infected fluid collections throughout the breast, and inability to obtain adequate drainage of infected material with a percutaneous approach. If the patient does not improve within 36 hours, then breast ultrasound is indicated to look for a fluid collection defining the presence of an abscess. Ultrasound showed a fluid collection underlying the site of erythema, connected with a larger fluid collection occupying the entire subareolar space, and having an estimated size greater than 10 cm. Because of large size and chronicity of symptoms, decision was made for surgical drainage. A radially oriented incision was made in the medial breast, 160 mL of pus was drained, abscess wall was debrided, and tissue sent for biopsy, which was benign. In cases of documented lactational abscess, consultation with a lactation consultant is appropriate preoperatively to help manage ongoing lactation postoperatively and to ensure proper lactation techniques to help correct problems that could predispose the contralateral breast similarly to mastitis and abscess. If the patient wishes to stop nursing, gradual rather than immediate cessation of lactation is preferred because of the ensuing engorgement that occurs with abrupt cessation of lactation, magnifying postoperative pain and possibly increasing the risk of milk fistula. If the infectious process is severe with extensive tissue necrosis, consultation with a plastic surgeon is advisable to help plan the surgical approach and optimize the cosmetic result. In the medial and lateral breast, this will be a radially oriented incision, whereas in the upper and lower central breast, this will constitute a transverse (antiradial) incision. The incision needs to be long enough that the resulting abscess cavity can be packed easily and the skin will not heal over before the depth of the wound has contracted. The incision is deepened in to the abscess cavity, and intraoperative cultures are obtained, with care to provide the culture specimen in the correct medium/container to allow both aerobic and anaerobic cultures. One week later, she underwent superficial incision and drainage in the emergency department. Ultrasound showed complex fluid collections in both upper quadrants and a third fluid collection in the deep upper inner quadrant. Pus extended throughout the central breast and in to the retromammary space, and tissue biopsy was benign. Dressing changes were performed in the operating room at 24 and 48 hours at which time a wound vac was placed. The wound granulated well by 2 weeks and showed dramatic contraction and healing by 4 weeks (C). Any necrotic overlying skin is debrided, and the wound is explored for loculations and tracts to ensure complete drainage. Vigorous irrigation and meticulous hemostasis are performed, and the wound is packed loosely with saline moistened gauze. Alternatively, do not remove extra healthy tissue; it is not necessary to excise back to normal-appearing tissue, as this will increase the tissue defect without any wound-healing benefit. If the tissue defect is large (greater than approximately 20% of the breast volume), it is advisable to have a plastic surgeon evaluate the wound early on to help with managing the long-term cosmetic result. Even large abscess cavities will usually heal with a good cosmetic result by secondary intention. Although excision of a small abscess and primary closure is tempting to avoid dressing changes, primary closure is contraindicated. Additional supportive care with ongoing lactation is also recommended as mentioned earlier. Clinical signs of acute inflammation Chapter 2 Drainage of Breast Cysts and Abscesses 35 should resolve within a week; if not, repeat ultrasound should be performed to look for any undrained fluid collections. All patients should undergo diagnostic mammogram after resolution of the breast abscess to establish a baseline mammogram. Plastic surgery consultation can be considered in the elective setting once the infection has resolved. Recurrent abscess can result from inadequate operative drainage or premature closure of the skin before healing of the deep tissues, which may be due to an incision that is too small or due to inadequate wound care. This is characterized by milk that drains directly from an open surgical wound in the lactating breast. Wound management is indicated to protect the skin, and moist dressings should be maintained in the subcutaneous tissue. Usually, the tissues will granulate, with spontaneous resolution of the milk drainage within 4 to 6 weeks. If a milk fistula persists beyond 6 to 8 weeks, then cessation of lactation from the involved breast is indicated. Lactational abscesses that require surgical drainage uniformly resolve with proper treatment, and cosmetic compromise is uncommon. These abscesses generally occur away from the nipple areolar complex but can be rapidly progressive. The usual microorganism is Staph aureus, but cultures and sensitivities should be obtained. Serial percutaneous aspiration is preferred for small abscesses in a stable patient. For those that fail percutaneous drainage or progress despite aspiration, operative drainage is indicated. Tissue biopsy of the abscess cavity is recommended for those cases that proceed to open drainage. Diagnostic mammography and ultrasound should be performed 3 months after resolution of the abscess to rule out any residual mass or findings suspicious for malignancy. Nonlactational abscesses that occur in the periphery of the breast should be treated similar to lactational abscesses with two primary differences: (1) broad-spectrum antimicrobial therapy is needed and (2) biopsy of the abscess cavity is strongly suggested to rule out cancer at the time of open drainage. Surgical management of recurrent subareolar breast abscesses: Mayo Clinic experience. These abscesses are strongly associated with a smoking history, with greater than 70% of affected patients being active smokers. If subareolar abscesses are untreated, they will spontaneously drain (usually at the vermilion border of the areola) and progress to chronic fistula. Antibiotic treatment must provide coverage for Staphylococcal species as well as for anaerobes. In the absence of culture results, suggested empiric regimens are minocycline and levofloxacin (Levaquin) or cephalexin and metronidazole. The patient should be seen weekly to assess improvement or sooner for progression of symptoms. Some authors recommend elective excision, whereas others recommend reserving operation for cases that recur. If there is any visible associated abnormality of the nipple (retraction, inversion, or a central crease), the likelihood of abscess recurrence is high, and the patient should be counseled regarding the option for elective excision of the abscess site and involved central nipple duct(s). If the abscess is pointing with near necrosis of the skin at the areolar edge, then incision and drainage under local anesthesia will facilitate drainage and provide material for bacterial culture, with prompt symptomatic relief for the patient. Combined antibiotic therapy is recommended as for early abscess, tailoring treatment to culture results. If the abscess is very small, has no fistula, and resolves with a single aspiration and antibiotic therapy, then operation can be omitted, but subareolar abscesses recur commonly without excision of the abscess cavity and associated obstructed nipple duct(s). For the rare patient who presents with multiple fistulae resection of the entire nipple areolar complex may be necessary, and preoperative consultation with a plastic surgeon is advised. The nipple stalk should also be rolled between the thumb and the forefinger to identify any masses or enlarged/thickened ducts, as well as discharge of pus from the nipple surface. In the setting of acute inflammation, ultrasound should be obtained to define whether an abscess is present. These imaging studies should focus particular attention to the subareolar and periareolar region.
Copper. Fildena.
- Osteoporosis. Taking copper in combination with zinc, manganese, and calcium might slow bone loss in postmenopausal women.
- Are there safety concerns?
- Anemia due to copper deficiency.
- Copper deficiency.
- Are there any interactions with medications?
- Wound healing, arthritis, and other conditions.
- What other names is Copper known by?
- Dosing considerations for Copper.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96868
Order fildena no prescription
These are usually made of a hydrophilic polymer that is cross-linked either by chemical bonds or by other cohesion forces such as ionic interaction erectile dysfunction drugs uk cheap 150 mg fildena visa, hydrogen bonding, or hydrophobic interactions. Hydrogels behave like an elastic solid in a sense that they can return to their original conformation even after a long-term loading. A hydrogel swells for the same reason as its linear polymer dissolves in water to form a polymer solution or hydrosol. From a general physicochemical standpoint, a hydrosol is simply an aqueous solution of a polymer. Many polymers can undergo reversible transformation between hydrogel and hydrosol. When a hydrogel is made by introducing gas (air, nitrogen, or carbon dioxide) during its formation, it is called a porous hydrogel. Dissolution of a polymer in a solvent is an entropy-driven process that happens spontaneously. A dry hydrogel is in its solid state and has the tendency to obtain more freedom as it goes in to solution. As the ion content of a hydrogel increases, two very strong osmotic and electrostatic forces are generated within the hydrogel structure. The presence of ions inside the gel and the absence of the same ions in the solvent trigger a diffusion process (osmosis) by which water enters the polymer structure until the concentration of the ion inside the gel and the solvent becomes equivalent. In fact, the polymer diffuses in to water to balance its ion content with the surrounding solution. Polymer chains carrying ions are charged either negatively (anionic) or positively (cationic). In either case, similar charges on the polymer backbone will repel each other upon ionization in an aqueous medium. This creates more spaces inside the hydrogel and more water can be absorbed in to its structure. Linking polymer chains to each other can do this, generating elastic forces and causing less entropy. The concept behind this is the osmotic pressure that is generated by either hydrophilicity (as in vinyl pyrrolidone) or ionic (as in In water Less hydrophilic In alcohol or salt solutions Low Mw High Mw More hydrophilic. Sodium starch glycolate (Explotab, Primojel, Vivastar P), cross-linked poly (vinyl pyrrolidone) (Crospovidone), and cross-linked sodium salt of carboxymethyl cellulose (Ac-Di-Sol, Croscarmelose) are widely used as a tablet and capsule disintegrant in oral dosage forms. Oros provides 24 hr controlled drug release that is independent of many factors such as diet status. The upper section contains drug and the lower section contains the osmotic agent either a salt or a water-soluble/swellable polymer. The membrane allows water or the aqueous medium to enter in to the osmotic agent compartment. In the presence of water, osmotic pressure pushes the bottom compartment upward which in turn forces the drug through a laser- drilled orifice on top of the tablet. Duros technology is utilized in implants that deliver drugs over a very long period. Depending on the nature of cross-linking, a hydrogel is classified as chemical or physical. Therefore, chemical gels will not dissolve in water or other organic solvent unless the covalent cross-links are broken apart. At least two different approaches can be used to form chemical gels, either by adding an unsaturated olefinic monomer carrying more than one double bond. In general, cross-linking through double bond is energetically favored as less energy is required to break a double bond than to react the functional groups. Cross-linked polymers of acrylic acid, sodium acrylate, and acrylamide have found extensive application in hygiene and agricultural industries as water absorbent polymers. Laser-drilled orifice Drug compartment Tablet coating Semipermeable membrane Osmotic compartment. Most chemical gels especially those made of hydrophilic chains can swell more in warmer solutions. On the other hand, some hydrogels made of relatively hydrophobic monomers shrink upon increase in temperature, and they are known as thermoshrinking chemical gels. The thermoshrinking hydrogels undergo thermally reversible swelling and deswelling. Modern or advanced pharmaceutical dosage forms utilize polymers for drug protection, taste masking, controlled release of a given drug, targeted delivery, increase drug bioavailability, and so on and so forth. Apart from solid dosage forms, polymers have found application in liquid dosage forms as rheology modifiers. They are used to control the viscosity of an aqueous solution or to stabilize suspensions or even for the granulation step in preparation of solid dosage forms. Major application of polymers in current pharmaceutical field is for controlled drug release, which will be discussed in detail in the following sections. In the biomedical area, polymers are generally used as implants and are expected to perform longterm service. This requires that the polymers have unique properties that are not offered by polymers intended for general applications. In general, the desirable polymer properties in pharmaceutical applications are film forming (coating), thickening (rheology modifier), gelling (controlled release), adhesion (binding), pH-dependent solubility (controlled release), solubility in organic solvents (taste masking), and barrier properties (protection and packaging). From the solubility standpoint, pharmaceutical polymers can be classified as water-soluble and water-insoluble (oilsoluble or organic soluble). The cellulose ethers with methyl and hydroxypropyl substitutions are water-soluble, whereas ethyl cellulose and a group of cellulose esters such as cellulose acetate butyrate or phthalate are organic soluble. The synthetic water-soluble polymers have also found extensive applications in pharmaceutical industries, among them polyethylene glycol, polyethylene glycol vinyl alcohol polymers, polyethylene oxide, polyvinyl pyrrolidone, and polyacrylate or polymethacrylate esters containing anionic and cationic functionalities are well-established. Physical Gels Hydrogen bonding, hydrophobic interaction, and complexation are three major tools in preparing a physical gel. The hydrogen is covalently attached to one atom, the donor, but interacts electrostatically with the other, the acceptor. Although its structure suggests an easy dissolution in water, a poly (vinyl alcohol) at molecular weight more than 100,000 g/mol is water insoluble. In order to dissolve the polymer, the hydrogen bonds need to be broken and that requires the solution to be heated up to 80 C to 90 C. Hydrophobic interactions are considered to be the major driving force for the folding of thermoresponsive hydrogels and globular proteins. The more hydrophobic groups within the hydrogel structure, the more temperature dependent the swelling will be. Once the solution temperature increases up to a certain point, these solutions become thicker by forming a gel. Complexation may happen between two oppositely charged groups of different polymer structures or via metal ions. In water, alginic acid with negatively charged groups and chitosan with positively charged groups can form a complex. The solubility of the complex is generally dependent on the pH of the dissolution medium and the pKa of the polymers. On the other hand, alginic acid carrying negatively charged carboxyl groups can form insoluble complexes with divalent and trivalent ions such as calcium, aluminum, and iron. Hydrogels either chemical or physical are also known as smart, intelligent, or responsive as they react to the environmental changes such as pH, temperature, salt concentration, salt type, solvent composition, or pressure. The unique properties of respon- Cellulose-Based Polymers Although cellulose itself is insoluble in water, its watersoluble derivatives have found extensive applications in pharmaceutical dosage forms. Methocel polymers including pure methylcellulose and hydroxypropyl-substituted methylcellulose display thermogelling property in water.
Purchase fildena 50 mg with amex
Short-term toxicity with the MammoSite has been minimal erectile dysfunction treatment stents buy fildena 100 mg amex, with mild erythema, pain, drainage, seroma, and ecchymosis seen occasionally and an infection rate of less than 10% with only the very rare infection necessitating balloon removal. After placing a balloon device there may be fluid that is trapped between the balloon and the lumpectomy cavity wall. Gentle breast massage can be used to evacuate this fluid through the track and out of the lateral stab incision. If the MammoSite is being placed in the operating room, sometimes the skin-to-balloon distance is inadequate. This can be solved by reopening the wound, excising the thin skin flaps, and reapproximating additional subcutaneous tissue before closing the skin. As the lumpectomy cavity heals, it will become progressively more fibrotic and less distensible, eventually disappearing entirely. This healing fibrosis may make symmetrical deployment of the MammoSite balloon difficult (may be less of an issue with the Contura (polyurethane) stiffer balloon or the bundled multicatheter devices). This healing also means that there is a finite amount of time between the lumpectomy surgery and the placement of the MammoSite or other brachytherapy device (usually 4 weeks). One of the advantages of brachytherapy is to get all the radiation therapy completed prior to the initiation of systemic therapy. Essentially, all cavities are irregular; however, the cavity walls can usually be pushed in to a sphere by the balloon devices. The bundled devices can accommodate the irregular walls and the treatment plan will compensate for the irregularity. The MammoSite Registry was taken over by the American Society of Breast Surgeons in November 2003. The registry has 1400 patients (1449 treated breasts) with a median follow-up of more than 5 years as of the end of 2009. This trend should continue since tumor size is continuing to decrease (thus allowing more women to meet selection criteria). The keys to successfully implementing a breast brachytherapy program include (i) interdisciplinary care with close coordination between the surgeon and the radiation oncologist, (ii) appropriate patient selection, (iii) intraoperative surgical planning which optimizes the postoperative device placement, and (iv) familiarity and knowledge of more than one device to choose the appropriate device for the individual patient. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Postoperative ultrasound-guided percutaneous placement of a new breast brachytherapy balloon catheter. Two-year outcome with the MammoSite breast brachytherapy applicator: factors associated with optimal cosmetic results when performing partial breast irradiation. Descriptions and outcomes of insertion techniques of a breast brachytherapy balloon catheter in 1403 patients enrolled in the American Society of Breast Surgeons MammoSite breast brachytherapy registry trial. Five-year results: the initial clinical trial of MammoSite balloon brachytherapy for partial breast irradiation in early-stage breast cancer. Tumoral foci are usually near the primary tumor, and residual microscopic disease occurring in the same quadrant as the resection is often the cause of local disease recurrence. Other studies with brachytherapy with longer follow-up demonstrated that partial radiation therapy can be performed safely with good local control and good cosmesis in selected patients. Convenience and Cost Shortening the course of radiotherapy from the standard 6-week regimen to a singledose intraoperative technique lowers costs and allows more convenience for patients. It also increases compliance to 100% and eliminates the attrition rate during standard radiotherapy because of side effects or logistical difficulties in traveling to the radiation facility (1). It may allow more breast conservation in rural geographic areas where radiation facilities or transportation are not readily available and where many women must undergo mastectomy because of these secondary issues. It avoids an incorrectly directed boost of standard radiotherapy because of lack of clips or seroma to define the lumpectomy cavity accurately. Radiation to the skin is also reduced by shielding and may be associated with improved cosmesis and possibly elimination of radiation-induced angiosarcoma. Elimination of Delay in Receiving Radiotherapy When radiation is delayed because of timing around chemotherapy, there is evidence to suggest increased local failure (3). Although data are conflicting, irradiating immediately renders arguments for or against giving chemotherapy first moot. The European Institute of Oncology trial limits participants to patients older than 48 years with unifocal small invasive cancer (maximum tumor diameter 2. Exclusion criteria for standard radiation also apply, such as collagen vascular disease and pregnancy, although a preliminary dose analysis of potential fetal radiation exposure in Milan showed negligible delivery to the fetus (9). When brachytherapy is used, the lumpectomy is performed in a standard fashion, either with or without preoperative needle localization. Several different machines are in use for delivering electron beams, and whereas previously these were stationary, currently most are portable. Mobile linear accelerators include Linac (Info&Tech, Rome, Italy), Novac7 (Hitesys Srl, Aprilia, Italy), Mobetron (IntraOp Medical Corp, Santa Clara, California), and Intrabeam (Zeiss, Inc, Oberkochen, Germany). These machines are portable electron delivery systems that can be moved in to the operating room. Electron beams are delivered at variable energies (3, 5, 7, and 9 eV) with maximum energy of 10 to 12 MeV to limit possible exposure to other operating rooms (10). It is a photon radiosurgery system that has been approved by the Federal Drug Administration for use in any part of the body since 1999 (11). Photon radiosurgery also uses electron beams, but these are used to generate X-ray photons at the tip of a wand-like instrument that is inserted in to the lumpectomy cavity. This results in delivery of radiation from the lumpectomy cavity outward, as with brachytherapy. The limit of this method is the superficial penetration of the radiation beam: only 2 mm of tissue surrounding the lumpectomy cavity receives the therapeutic dose of 20 Gy. For all mobile linear accelerators, the lumpectomy or quadrantectomy is initially performed as usual. A skin incision is made, then dissection carried through subcutaneous fat and breast parenchyma to reach the tumor, which is removed with a 1 to 2 cm margin of normal surrounding tissue. Skin flaps are raised from the underlying breast tissue for a few centimeters circumferentially to allow retraction away from the radiation source. Breast parenchyma is then lifted free from the pectoralis muscle to allow insertion of a protective aluminum-lead disk over the pectoralis. Once this is inserted, the breast parenchyma is temporarily closed over the disk with sutures and the skin is retracted. Radiation beams are collimated, or aligned, by a 5-mm-thick Perspex tube that has two parts: the sterile portion is placed in the operative field by the surgeon before connection to the distal portion, which is managed by the radiation oncologist. Electron beams are delivered perpendicular to the tissue for 2 minutes, with electron energies corresponding to the distance of penetration. After radiation delivery, the tube is removed and the lumpectomy incision closed as usual, with or without breast parenchymal closure. The main difference between this technique and the electron beam therapy is that the skin and chest wall do not have to be protected from the radiotherapy source because radiation is delivered internally to externally and diffuses sufficiently by the time it reaches the skin and muscle, thus avoiding significant toxicity (12). A 1 cm margin of normal breast tissue between the radiation source, skin, and chest wall is preferred. The sterile applicator is inserted in to the lumpectomy cavity and the tubing connected to the radiation delivery source. The skin edges are covered with sponges or towels to protect them from the radiation source. Computer-calculated dosimetry is used to determine the dwell time of the radiation source within the catheters and the total treatment time. The radiation source is delivered through the tubing in to the applicators and left in place for the prescribed time. When radiation delivery is completed, the source is removed and the lumpectomy incision closed as usual. The main advantages are convenience for the patient, lower cost, greater accuracy in delivery with less radiation to the skin and contralateral breast, elimination of delay in administering radiotherapy, and the possibility of expanding indications for radiation to previously irradiated patients. Early trial results show comparable local recurrence rates, but follow-up is limited. The preference of the European Institute of Oncology, Milan has been to perform quadrantectomy to remove a generous portion of tissue and thus reduce positive margin rates. In the brachytherapy trial at Memorial Sloan-Kettering Cancer Center, the specimen is examined grossly after sectioning for any close margins.
Order 100 mg fildena amex
Examples of convective mixers are ribbon blenders erectile dysfunction causes psychological buy fildena discount, screw blenders, planetary blenders, and high-intensity mixers. If a simple blend of excipients and active ingredients does not have the physical, chemical, or mechanical properties needed to achieve the manufacturing and performance requirements, the blended powders may be further processed using granulation methods. A direct blend, often referred to as a direct compression formulation, is advantageous and often preferred over granulated powders because it requires fewer steps for manufacturing and is therefore more cost-effective. Granulation is the process of particle agglomeration and size enlargement of powdered ingredients to achieve desirable processing properties such as improved powder flow or compression. Within the pharmaceutical industry dry granulation and wet granulation methodologies are most often used if a direct blend is not suitable. Wet granulation is achieved by mixing a granulating fluid, often water, together with other blended components to achieve a wet mass that forms larger agglomerates called granules. Once the desired granule growth has been achieved, commonly referred to as a granulation endpoint, the wet massing process is stopped and the granules are then dried. As the drying occurs, ingredients which were dissolved in the granulation fluid form solid bridges that hold the particles together. The wet granulation process has a number of advantages related to improved processability but its disadvantages include exposure of the formulation components to granulating liquid and exposure to the elevated temperatures necessary to dry the wet granules. Wet granulation may be carried out in high shear equipment or alternatively utilize fluid bed technology. The properties of the granules formed depend on the properties of the individual materials used and the process and the process parameters that are used in granulation. Dry granulation is achieved by compressing powdered materials in to dense, cohesive compacts which are then milled and screened to produce a granular form of material with desirable particle size characteristics. The compaction process in dry granulation may be achieved in a continuous fashion using what is known as roller compaction. Roller compaction is the process of compressing powder blend to produce a solid ribbon between two rollers. An alternative and less commonly used method is to compress powders in to large tablets, called slugs, which are then milled and screened. Among the advantages of dry granulation is that the materials are not exposed to granulation fluid or the high temperatures required to dry the granulated material. In the manufacture of solid dosage forms, it is sometimes necessary to include a wet granulation step in the manufacturing process as described above. Drying is undertaken to remove excess water (or other granulation liquid) from the solid granules by evaporation. The drying process is designed to reduce the moisture content to an acceptable value. Among the most commonly used in the pharmaceutical industry are direct heating methods where heat transfer is accomplished by direct contact between the wet solid mass and heated air. An example of a static method of drying is tray-drying where the granulation is placed on a tray that is then placed in an oven and drying takes place. An alternative and more common method that is conducive to large-scale manufacturing is to physically move the moist granulation with heating to cause evaporation. The most commonly used method of drying is fluidized bed drying where the granulation is fluidized in heated air. A separate blending step, called the lubrication step, is described here because it is a very frequently used unit process. As with the other unit processes, the properties of the lubricant and the process parameters must be carefully assessed and characterized because an inappropriately performed lubrication step can have a significant negative impact on dosage form performance. The commonly used lubricants magnesium stearate or stearic acid are very water insoluble. Incorporation of an excessive amount of one of these ingredients or excessive mixing has been shown to decrease the dissolution rate of the final dosage form. Following the blending, granulation, and lubrication steps, the formulation is ready for compression in to a tablet. A wide variety of tablet dosage forms with a remarkable range of performance characteristics can be prepared with the proper selection of formulation ingredients and processing. The seeming simplicity of the tablet dosage form belies the remarkable flexibility and creativity this technology offers as a sophisticated drug delivery device. Powder compression in to tablets is the application of pressure to the formulated powder to achieve a reduction in volume and the generation of strength within the compacted material to form an intact tablet. Tablet tooling consists of a lower punch which snugly fits in to the tablet die from below and an upper punch which can enter the die from above. The die serves to hold the formulated powder in place when the lower punch is in place, and the upper and lower punches are forced together to compress the powder. Powder compaction can be done using a small, hand-operated press but, of course, in a large-scale manufacturing environment, high-speed tableting machines are used to produce thousands of tablets per minute. The first step (Stage 1) is the die filling step in which powder is moved in to the die. The lower punch position holds the powder in the die and determines the amount of powder that the die will hold. The compression process begins in the second step (Stage 2) as the upper punch is. In the third stage of compression (Stage 3), significant particle deformation and possibly particle fracture occur as the powder further consolidates in to a cohesive mass. The decompression stage (Stage 4) begins as the upper punch force is reduced and the upper punch is removed. During the decompression stage some of the elastic deformation that occurred during compression results in some tablet expansion. If the formulation is properly designed, the final stage (Stage 6) results in the ejection of an intact tablet that has the desired strength and performance characteristics. Tablet strength is influenced by the formulation components, the processing used to make the formulation, and the process of forming the compressed tablet. The resistance of tablets to chipping, abrasion, and breakage depends on tablet hardness. Tablet hardness is used as a manufacturing control tool and hardness values are often determined throughout a tablet manufacturing lot. If tablet hardness values vary, adjustments to the tablet machine can be made to ensure that the tablet hardness remains within the accepted range. Tablet hardness values should be high enough to ensure satisfactory appearance and tablet strength to withstand further tablet processing and handling but not so high that the dosage form will fail performance criteria such as disintegration or dissolution. Friability Tablets must be hard enough to withstand the agitation and stresses that occur during manufacturing, coating, packaging, shipping, and patient use. Tablet friability is a measure of the ability of the tablets to withstand stresses. Tablets are placed in a 12-inch diameter drum which rotates for a set period of revolutions, typically 100. A shaped arm lifts the tablets and drops them half the height of the drum with each revolution. Disintegration One simple measure of the ability of a compressed tablet or capsule to release drug is the disintegration test. The disintegration time is the time it takes for a dosage form to break apart upon exposure to water with mild agitation. The mechanical strength 590 For conventional immediate release tablets, disintegration times may range from less than 1 min to as much as 5 to 15 min. The disintegration time is markedly affected by formulation ingredients and processing. However, disintegration time does not necessarily bear a direct relationship to in vivo release of drug from a dosage form. To be absorbed, the drug substance must be in solution and the disintegration test only measures the time required for the tablet to break up in to particles or for a capsule to disperse its contents.