Purchase cymbalta 40 mg
Since 1965 anxiety symptoms go away buy cheap cymbalta 60mg online, several reports of an association of topical chloramphenicol and the development of aplastic anemia have been published (Rosenthal and Blackman, 1965; Davidson, 1974; Carpenter, 1975; Abrams et al. There are several possible routes by which chloramphenicol might enter the body after topical application to the eye: by absorption through the nasal mucosa after passing through the nasolacrimal ducts; by absorption into the aqueous humor; or by absorption from the gut after swallowing tears that contain the drug (Carpenter, 1975; Fraunfelder et al. However, a study of 40 patients given ophthalmic chloramphenicol failed to detect the drug with highperformance liquid chromatography in serum after 2 weeks of treatment (Walker et al. In many of the cases ascribed to ophthalmic administration, topical administration was continued for many months, but this, like bone marrow aplasia after parenteral administration of chloramphenicol, is not a prerequisite because this complication is idiosyncratic. Although some consider it "proven" that the ophthalmic preparation of chloramphenicol is a cause of aplastic anemia, this is not the case because the occurrences may be coincidental or other drugs known or unknown may have been the causative agents (Wiholm et al. Establishing clear causation in instances of suspected drug toxicity can be difficult. Given the rarity of aplastic anemia when chloramphenicol is administered systemically, the confirmation of a definite relationship between ophthalmic chloramphenicol and aplastic anemia would require a very large number of patient observations (Fraunfelder and Bagby, 1983). Several epidemiologic studies have failed to show a connection between topical chloramphenicol and aplastic anemia (Besamusca and Bastiaensen, 1986; Lancaster et al. Although there are no convincing data proving or disproving this connection, it seems most 6. Adverse reactions and toxicity 1527 likely that the risk is either absent or less than one per million treatment courses (Laporte et al. When selecting topical ophthalmic antibiotic therapy, topical chloramphenicol should be avoided in patients with a previous history or family history of drug-related hemopoietic toxicity. There is some confusion over the association of liver disease, aplastic anemia, and chloramphenicol administration. A case series in the early 1970s described five patients who developed aplastic anemia after receiving chloramphenicol during the preicteric phase of hepatitis, presumably of viral etiology (Hodgkinson, 1973). There are numerous reports of an association of liver damage with aplastic anemia and chloramphenicol administration (oral and intravenous); usually chloramphenicol administration precedes the hepatitis and pancytopenia (Bennett and Lucas, 1979; Casale et al. The etiology of this syndrome, which is usually fatal, is not clear, and there is no evidence to indicate that the liver damage is caused by a hepatitis virus or, for that matter, by chloramphenicol. If abnormalities of liver function appear during chloramphenicol administration, the drug should be discontinued because in patients with this syndrome, liver function test results became abnormal before or concomitant with hemopoietic toxicity (Casale et al. The exact mechanism is unknown but is most likely to be due to direct inhibition of mitochondrial protein synthesis (Yunis, 1988). In addition, there is evidence that chloramphenicol suppresses the activity of ferrochelatase, an enzyme that normally catalyzes hemoglobin synthesis in the mitochondria of bone marrow erythroid cells (Manyan et al. Chloramphenicol in concentrations that are achieved in serum during therapy also inhibit bone marrow colony formation in vitro (Howell et al. The administration of large doses of chloramphenicol for several weeks is associated with reduced iron utilization for hemoglobin synthesis, vacuolization of erythroblasts, a low reticulocyte count, and thrombocytopenia and leukopenia with vacuolization of marrow granulocyte precursors (Scott et al. In one study, these changes occurred regularly when the serum levels of chloramphenicol were 25 mg/l (Scott et al. Similar changes occurred more rapidly in patients with hepatic disease who developed high chloramphenicol serum levels more rapidly. Such side effects are reversible after cessation of the drug; the serum iron falls abruptly, reticulocytosis appears, and the bone marrow and the peripheral blood return to normal within a few days. In some cases, erythropoietic recovery may occur even if chloramphenicol is continued. In one study of children with septic arthritis receiving 6 weeks of chloramphenicol, there was a steady increase in serum hemoglobin concentrations after initiation of treatment (Peek et al. Chloramphenicol prevents the expected reticulocyte response in patients with pernicious anemia treated with vitamin B12 and the expected response to iron in patients with iron deficiency anemia. These phenomena are probably explicable in terms of the known mode of action of chloramphenicol on rapidly multiplying cells (Weisberger et al. Hemolytic anemia has been described in patients with glucose-6-phosphate dehydrogenase deficiency who are treated with chloramphenicol (McCaffrey et al. Paradoxically, a patient with chronic neutropenia has been treated with chloramphenicol to stimulate neutrophil maturation and release; the drug was used for this purpose for more than 12 months (Adams and Pearson, 1983). In vitro, chloramphenicol in therapeutic concentrations markedly depresses leukocyte migration (Forsgren and Schmeling, 1977) and may suppress antigen-induced lymphocyte blastogenesis (DaMert and Sohnle, 1979); in high concentrations, such as 200 mg/l, it also impairs phagocytosis (Melby and Midtvedt, 1977). There have been conflicting epidemiologic studies regarding chloramphenicol and leukemia. However, a subsequent study in the same area failed to show a similar association (Zheng et al. Gray (baby) syndrome this is a type of circulatory collapse that can occur in premature and newborn infants and is associated with excessively high serum levels of chloramphenicol (Sutherland, 1959). In one study, chloramphenicol was given to 61 premature infants in high doses ranging from 100 to 165 mg/kg daily (Burns et al. Whether the mortality would be as high with modern intensive care and extracorporeal support is unknown. A potentially reversible alteration of myocardial function accounts for some, if not all, of the syndrome (Biancaniello et al. This syndrome probably occurs mainly in neonates because they have impaired glucuronidation of chloramphenicol in the liver and reduced renal excretion of both free chloramphenicol and its succinate ester. She developed severe shock, cyanosis, and coma 5 hours after the last dose, but made a full recovery. The infant recovered after an exchange transfusion and supportive care (Thompson et al. In more recent times, the gray syndrome in infants has usually has been due to accidental overdosage with chloramphenicol (Kessler et al. In these reports of infants with toxicity, serum chloramphenicol levels ranged from 98 to 180 mg/l; but the gray syndrome has been reported with a serum level as low as 40 mg/l (Glazer et al. Even when a reduced dosage of intravenous chloramphenicol succinate is used in infants, resultant serum levels are erratic and dosage should be monitored according to serum levels to avoid toxicity (see section 5b, Drug distribution-Intravenous administration). Surprisingly, high serum concentrations of chloramphenicol sometimes occur without signs of toxicity. For instance, a 10-week-old boy received an accidental overdosage of chloramphenicol. The serum level reached 130 mg/l and then declined to 0 over the next 40 hours without clinical evidence of toxicity (Stevens et al. In a review of 64 neonates given chloramphenicol, 10 exhibited clinical features attributed to toxicity (Mulhall et al. Of these 10, 1 received an accidental overdose and 9 received the prescribed dose, although in 6 this was greater than recommended. Peak serum concentrations in these 10 infants ranged from 28 to 180 mg/l and trough levels from 19 to 47 mg/l. In 27 other neonates, serum chloramphenicol levels above the therapeutic range were observed (2 had received a 10-fold overdose) without signs of toxicity; in seven of these it was in excess of 50 mg/l. Toxicity was not related to the duration of the high serum level, but seemed to be more common in infants younger than 9 days. Infants with high serum chloramphenicol levels and no clinical abnormalities can be safely observed after discontinuation of the drug. Associated liver dysfunction may be an increased risk factor in such children (Stevens et al. Chloramphenicol intoxication in infants with the features of the gray syndrome has been treated by exchange transfusion with variable results (Kessler et al. Optic neuritis this complication has been described in a small number of patients treated with chloramphenicol, resulting in optic atrophy and blindness (Cocke et al. Most of these patients were children with cystic fibrosis receiving prolonged chloramphenicol treatment for pulmonary infection.
Syndromes
- Fatigue
- In the past, most patients with heart valve problems such as mitral stenosis were given antibiotics before dental work or invasive procedures, such as colonoscopy. The antibiotics were given to prevent an infection of the damaged heart valve. However, antibiotics are now used much less often before dental work and other procedures. Ask your doctor whether you need to use antibiotics.
- Urinalysis
- Painkillers to reduce headache, muscle pain, and spasms (narcotics are not usually given because they increase the risk of breathing trouble)
- Occasional passage of very large stool that almost blocks up the toilet
- Headache
- Fluid (plasma or serum)
- Headache
- Has a new rash or bruises appear
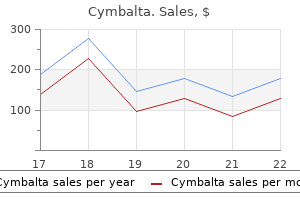
Buy 30mg cymbalta mastercard
In patients with severely reduced renal or hepatic function anxiety symptoms overthinking order cymbalta online pills, the serum halflife is somewhat prolonged (Avant et al. In neonates, the serum halflife is dependent on gestational age, body weight, and chronologic age. The serum halflife after topical administration of clindamy cin is primarily influenced by the cutaneous absorption rate. The elimination halflife of clindamycin vaginal ovules and vaginal cream had an average of about 11 and 14 hours, respectively (Borin et al. These authors reported low systemic absorption of clindamycin from the vaginal cream (about 4%), but systemic absorption averaged 30% with the clindamycin phosphate ovule. A single 150mg oral dose of clindamycin hydrochloride in adults results in peak serum concentrations in the range of 2. Peak serum concentra tions after administration of a single 300mg dose of clinda mycin hydrochloride results in 3 mg/l, and 450 mg produces about 4. The ester clindamycin palmitate hydrochloride is also well absorbed from the gas trointestinal tract (Campbell et al. The average serum concentration after administration of 150 mg of clindamycin palmitate ester produces slightly lower concentrations com pared with concentrations after administration of clinda mycin capsules (Dhawan and Thadepalli, 1982; Keusch and Present, 1976). Orally administered clindamycin palmitate ester is more slowly absorbed in adults than in children 1480 Clindamycin and Lincomycin (Dehaan and Schellenberg, 1972). The presence of food in the stomach somewhat delays but does not does significantly affect the absorption of both clindamycin hydrochloride and clindamycin palmitate (Dehaan et al. Clindamycin phosphate, the bioinactive ester, is rapidly hydrolyzed to the active form in the serum. After a 300mg intramuscular dose of clindamycin phosphate, the mean peak serum concentration ranges of 4. Fass and Saslaw (1972) also reported that diabetic patients tended to have lower serum clindamycin concentrations after intra muscular administration. Clindamycin phosphate 300 mg given as a 30minute intravenous infusion resulted in a mean peak serum level of 14. After intravenous admin istration of higher doses-900 mg every 8 hours and 1200 mg every 12 hours-clindamycin mean peak serum concentra tions have been reported to range between 12. Of concern, diarrhea associated with topi cal clindamycin has been reported in 3 patients-1 noted to have an inflamed mucosa on proctoscopy (Voron, 1978). This would seem to indicate that clinically significant amounts of clin damycin may be absorbed in some patients after topical administration. Topical clindamycin is widely used in the vagina in women with lower genital tract infections. It is interesting to note that systemic absorption of clindamycin appears to be slower in women with bacterial vaginosis than in healthy women. Systemic bioavailability of clindamycin after daily adminis tration of 100 mg of clindamycin (2% vaginal cream) is low, ranging from 1. The average peak serum concentration in these patients on day 3 was reported to be 0. A clindamycin phos phate 2% singledose vaginal cream formulation has been developed and provides release of clindamycin equivalent to seven daily doses of clindamycin phosphate 2% vaginal cream (Levinson et al. The absorption from clindamycin phosphate ovules was documented to be 30% (range 6. After intravaginal administration of clindamycin phos phate ovules, systemic absorption was noted to be approxi mately sevenfold greater than after administration with the 2% clindamycin phosphate vaginal cream. The first mention of intraperitoneal administration of clin damycin occurred in 1984 (Cohen and Bailey, 1984). Some of the initial investigations stated that the activation of clin damycin phosphate to the biologically active form was mini mal in peritoneal fluid, and therefore recommendations were that clindamycin should not be administered intraperitone ally (Eng et al. Other researchers found the opposite and reported significant activation when the clindamycin phosphate was admixed with the dialysate fluid and administered intraperitoneally (Chang et al. They also reported that the clindamycin phosphate was not fully acti vated to clindamycin in the dialysate. After five exchanges with 2 l of fluid containing 300 mg of clindamycin phosphate per liter, they reported a serum concentration of 5. The addition of antibiotics to bone cement has been widely used in revision surgery of infected arthroplasties and in the treatment of other joint infection or noninfected revi sions (Hinarejos et al. Clindamycin has been suggested as a possible antibiotic to be used in such instances because it has been reported to have release char acteristics from bone cement that are superior to gentamicin (Hill et al. They evaluated the antimicrobial effect of gentamicin plus clindamycin against a collection of 38 clinical isolates, including 16 gentamicinresistant isolates. Gentamicinloaded bone cement alone had an antimicrobial efficacy against 58% of the 38 bacterial isolates, whereas 68% of the isolates were affected by bone cement loaded with a combination of clindamycin and gentamicin. Clindamycin was documented to have the best elution profile, with the highest granulation tissue, bone, and seroma, concentrations. Ocular implants have been developed to improve the ocular pharmacokinetic of the drugs. Unfortunately, most antibiotics, when applied topically in burn patients, do not penetrate burn eschar in therapeuti cally relevant concentrations. These authors reported that increas ing clindamycin phosphate liposomal lipid concentration from 20 to 100 mM resulted in decreased penetration by approximately two times. The authors speculated that these data demonstrated that increasing the lipid concentration decreased the penetrance of clindamycin through burn eschar, as well as resulting in an increase in its permeation lag time. In addition, these authors speculated that deposi tion of nanoliposomes formulations in the burn eschar increases clindamycin deposition in the burn eschar. Drug distribution Clindamycin is generally well distributed throughout the body (Table 85. Clindamycin penetration into the central nervous system is considered erratic (less than 3%) (Picardi et al. Even when the meninges are inflamed, cerebral spinal fluid concentrations are low, ranging from 0. These low concentrations in the cerebral spinal fluid are, however, adequate for the treatment of Toxoplasma encephalitis (Gatti et al. Six hours after the injection, the average concentrations of clindamycin were 110. These authors also reported their experience treating pediatric patients with suppurative ocular infections. They reported positive out comes in 11 of 13 patients treated with 10 mg of clindamycin palmitate per kilogram per day. They also reported concen trations of clindamycin in the human eyelid ranging from 0. Body site Saliva Sputum Pleural fluid Eyelid tissue Tonsillar surface fluid Gastric juice Bile Local concentration Similar to serum concentration 0. Reference Keusch and Present, 1976 Mitchell, 1970; Raeburn and Devine, 1971 Fass and Saslaw, 1972; Panzer et al. Another group of Japanese investiga tors described their experience in treating 22 patients (12 men and 10 women) with clindamycin (Oishi et al. Fifteen of the patients responded well to therapy with only minimal side effects (Oishi et al. Concentrations of clindamycin in the saliva are similar to those found in serum (Keusch and Present, 1976). Peak con centrations in crevicular fluid are also reported to be similar to that found in serum, 2.
Buy cymbalta line
Routine Susceptibility Fidaxomicin is a narrow-spectrum agent that has been demonstrated to be selectively active against Gram-positive anaerobes (Table 88 anxiety medication over the counter cheap 40mg cymbalta overnight delivery. Propionibacterium and Lactobacillus) and Peptostreptococcus, and is poorly active against anaerobic Gram-negative bacilli (Goldstein et al. Enterobacteriaceae, Pseudomonas, Campylobacter, Helico bacter, Haemophilus, Bacteroides, Fusobacterium, Porphyro monas, Prevotella, and Veillonella) exceed 32 mg/l to 64 mg/l (Goldstein et al. It is interesting to note that of various Clostridium species tested, Clostridium bolteae (7), Clostridium clostridioforme (4), Clostridium innocuum (9), and C. Consequently, fidaxomicin has a low ecologic impact on the intestinal microbiome (Tannock et al. Unfortunately, fidaxomicin is not mentioned and therefore breakpoints are not provided. There was no evidence of reduced susceptibility to fidaxomicin, consistent with other studies (Freeman et al. The isolates belonged to 37 different ribotypes, but no differences were found among ribotypes, although type 027 was not present. In vitro studies indicate a low frequency of spontaneous resistance to fidaxomicin in C. Another in vitro study using site-directed mutagenesis revealed that an isolate of C. Other reported laboratorygenerated mutations included Arg89Gly, Gln1074His, Val1143Gln, and Val1143Asp (Seddon et al. The only clinical isolate with reduced susceptibility to fidaxomicin was obtained from a patient with recurrence of diarrhea 6 days after cure with fidaxomicin (Goldstein et al. The clinical significance of the association of resistance with drug exposure is unclear. Similar mutations in the homologous positions in other bacterial species that demonstrate reduced susceptibility to lipiarmycin, a related macrocycle compound, have been reported (Gultieri et al. Fidaxomicin and rifamycins are both inhibitors of bacterial transcription, but fidaxomicin acts at an earlier step in the transcription initiation pathway (Babakhani et al. The unique target site of fidaxomicin may explain its limited spectrum of antimicrobial activity, because subunits differ among bacterial species (Wostem, 1998). However, it has not been tested or proven that the differential spectrum of fidaxomicin or lipiarmycin between bacterial species is due to differences between subunits. These populations will have to be closely monitored during postmarketing experience. Given the limited data and the difficulty in drawing conclusions regarding different stages of hepatic impairment, caution should be advised also in patients with moderate hepatic impairment. Newborn infants and children the dose, safety, and efficacy of fidaxomicin in children have not been established, but studies are underway (ClinicalTrials. When administered orally, fidaxomicin is minimally absorbed and is excreted almost entirely through the feces. Pregnant and lactating mothers No prenatal or postnatal development studies have been performed. No adverse effects have been reported on fertility or early embryonic development and embryo-fetal development, 5. A phase I study with 150, 300, and 450 mg of fidaxomicin per day revealed maximal plasma concentrations near or below the limit of quantification (5 ng/ml) after 10 days of treatment (Shue et al. A single-center, open-label, randomized, two-period, crossover study was performed to determine the pharmacokinetics and the effect of food on the bioavailability of fidaxomicin in six healthy subjects (Lewis and Sears, 2011). Inter-individual variability in systemic exposure to fidaxomicin was generally > 25% in healthy volunteers. Peak concentrations in the feces are generally observed 24 hours after single oral doses, and concentrations remain detectable in the feces for up to 5 days. The main components of the elimination of systemically available fidaxomicin have not been identified. In a preclinical study, the volume of distribution at steady state was found to be less than or equal to body water, suggesting that fidaxomicin does not extensively distribute away from body water (Korgaonkar et al. Fidaxomicin did not cause any adverse effects after multiple dosing when administered orally to rats, monkeys, or dogs. In rats and monkeys the toxicity of fidaxomicin was evaluated at oral doses up to 90 mg/kg for 28 days, and in dogs at oral doses up to 9600 mg/day for 3 months. Fidaxomicin has a low bioavailability, and several different vehicles have therefore been explored in order to increase the systemic exposure. No treatment-related effects were seen in rats, rabbits, and dogs when the drug was administered as a single dose of up to 200 mg/kg intravenously. The only deaths reported were after intravenous administration of 200 mg/kg to rats; these deaths might have been due to a precipitation of fidaxomicin in the vasculature (Korgaonkar et al. In two double-blind placebo-controlled phase I studies and one open-label dose-finding clinical study, none of the adverse events were considered to be drug related (Shue et al. In one of these studies, more laboratory abnormalities were observed in the fidaxomicin arm, mostly not attributed to study medication, except for one subject who developed elevated aminotransferases up to 6. Three of these patients reported a macrolide allergy, suggesting that there may be cross-reactivity between fidaxomicin and other macrolides, even though the central ring of fidaxomicin differs from that of the other macrolides. An important adverse effect of all antibiotics that reach the intestinal lumen is damage to the intestinal microbiota. Using quantitative culture, the count of Bac teroides group bacteria, a major constituent of the intestinal microbiota, in stool was shown to decrease significantly during treatment with vancomycin, whereas it did not change during treatment with fidaxomicin (Louis et al. This was also true for Clostri dium coccoides group and Clostridium leptum group bacteria, although the difference did not persist after the end of treatment. Another argument for the sparing of the colonization resistance by fidaxomicin as opposed to vancomycin was made in a substudy of the same trial, which showed that colonization by vancomycin-resistant enterococci and Candida species occurred less frequently with fidaxomicin treatment (Nerandzic et al. The main clinical problem in this infection is its propensity to recur after remission of symptoms. Metronidazole leads to a later remission of symptoms than vancomycin, so metronidazole is often reserved for less severe infections. Double-blind randomized controlled trials of fidaxomicin, modified intention-to-treat analyses. In a pooled modified intention-to-treat analysis of both trials, fidaxomicin led to remission of symptoms in 88% of cases, versus vancomycin in 86% of cases (Crook et al. More important, fidaxomicin led to significantly fewer relapses than did vancomycin. In the same pooled analysis, 14% of subjects in the fidaxomicin group experienced a relapse, versus 26% in the vancomycin group (Crook et al. It is unclear whether the microbiota-sparing quality of fidaxomicin is still of added value over vancomycin in patients with multiple relapses, who may have already sustained too much damage to their intestinal microbiota. Post hoc subgroup analyses of both trials were done for patients with markers of severe disease, older age, first episode versus first relapse, cancer, renal failure, use of concomitant antibiotics, and infection with the epidemic strain of C. In this subgroup, the percentage of relapses was not significantly different between those treated with fidaxomicin and those treated with vancomycin (Cornely et al. Moreover, in one of these trials, more relapses occurred with fidaxomicin than with vancomycin (Louie et al. It is therefore unclear whether fidaxomicin is of added value over vancomycin in patients infected by this strain. Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Comparative microbiological studies of transcription inhibitors fidaxomicin and the rifamycins in Clostridium difficile. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Risk estimation for recurrent Clostridium difficile infection based on clinical factors. Pan-European longitudinal surveillance of antimicrobial resistance among prevalent Clostridium difficile ribotypes. Frequency and characterization of spontaneous lipiarmycin-resistant Enterococcus faecalis mutants selected in vitro.
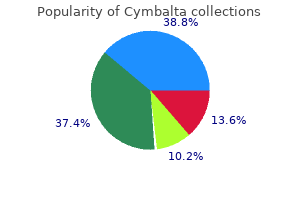
Purchase cymbalta in united states online
In patients with anuria or severe uremia (creatinine clearance less than 15 ml/min) anxiety symptoms preschooler buy 40 mg cymbalta amex, a full loading dose may also be given, followed by half this dose once or twice daily. Bergan and Brodwall (1976) consider that because of the possibility of an accumulation of sulfonamide metabolites, CoT should not be used in patients with a creatinine clearance of less than 15 ml/min, unless regular determinations of total sulfonamide serum levels can be made. Both drugs are removed during continuous renal replacement therapy, and patients undergoing this procedure should receive a full dose before and after dialysis (Craig and Kunin, 1973b; Curkovic et al. In patients receiving hemodialysis or peritoneal dialysis, dosing should be similar to that in a patient with a creatinine clearance of < 15 ml/min, with recent evidence suggesting that hemodialysis removes some but not all CoT (Clajus et al. Some authors have suggested full dosing for 48 hours then dose reduction based on clinical response (Paap and Nahata, 1989). Even in patients with severe renal failure, urinary levels of both drugs usually exceed those required for the eradication of most urinary pathogens. Pharmacokinetics and pharmacodynamics 1641 synergy against common urinary pathogens (Bushby, 1969; Adam et al. The authors suggested that increased doses or decreased dosing intervals are required when giving CoT to such patients, although specific doses will depend on the age and body weight of the patient. More important, however, is clinician awareness of polypharmacy in the setting of CoT use, because concurrent diuretic, antihypertensive, vitamin K antagonist, and sulfonylurea therapies are known to accentuate CoT-associated metabolic or electrolyte abnormalities (Antoniou et al. In children with biliary atresia, kinetics of both components of CoT can vary markedly, and if used in this population, levels should be measured (Lares-Asseff et al. Because CoT may interfere with folate metabolism, regular full blood examinations should be performed on patients receiving long-term therapy and those who are predisposed to folate deficiency. The pharmacokinetics of CoT are not impaired by any disturbance of liver dysfunction (Brown, 2014; Vree and Hekster, 1987). Thus, no dosage adjustment is considered necessary for patients with impaired hepatic function, and long-term oral CoT prophylaxis has been used successfully in patients 5. The serum half-life of the drug is about 13 hours, and detectable serum levels are still present 24 hours after a dose of 160 mg (Bushby and Hitchings, 1968). After a 1200-mg oral dose, a peak serum level of about 60 g/ ml of the active drug is attained 2 hours after administration, which persists for about 6 hours. The administration of these drugs in a 1:5 ratio as CoT results in their serum levels being in a ratio of about 1:20 to 1:30 when their peak levels are reached at about 2 hours. After peak levels, this ratio falls gradually to between 1:10 and 1:20 at the end of the dosage interval. In patients with impaired renal function, the ratio at the time of peak serum levels changes from 1:20 to 1:10 after 12 hours (Bergan and Brodwall, 1976). The 1:20 to 1:30 ratio with conventional CoT doses also applies when the combination is given in high doses frequently. Mean serum trimethoprim (a) and sulfamethoxazole (b) levels after an oral dose of 240 mg of trimethoprim and 1200 mg of sulfamethoxazole. Wide intersubject variation in the serum concentrations was noted within the initial 24 hours of therapy, such that in some patients this dose would not guarantee therapeutic concentrations against P. Bushby and Hitchings (1968) studied serum levels in six adult volunteers after an i. This does not differ significantly from the half-life of this drug after oral administration. These peak levels were similar in all age groups, although dosages per weight were higher in children than in adults. In 12 patients, peak serum levels after oral administration of CoT were compared; those after i. This differs from the serum ratio of these two drugs of about 12:1 after multiple doses, which Grose et al. In animals, tissue levels are usually greater than those in serum, except in brain, skin, and fat. Particularly high tissue levels have been found in the kidney and liver (Craig and Kunin, 1973a). Furthermore, it appears to concentrate in renal cysts in patients with polycystic kidney disease (Schwab and Weaver, 1986). In general, these ratios did not change with time after the dose or with prolonged dosing with CoT. Only lipid-soluble antibiotics are able to cross epithelial membranes, and only the unchanged (un-ionized) fraction of the drug is able to pass into prostatic fluid, because the ionized or charged fraction is 5. The degree of ionization of a molecule of a drug is determined by its dissociation constant (pKa), and drugs mainly in the un-ionized state have a high pKa. Therapeutic levels of CoT have been detected in prostatic tissue obtained at prostatectomy of patients given CoT (Leading article, 1983b). CoT penetrates bacterial biofilm only slowly but is still able to show some antibacterial activity, at least against E. The mean tissue/serum ratios were equivalent in the high- and low-dose CoT groups: 1. Approximations of the pharmacodynamics have been applied to the estimation of CoT breakpoints for N. These data suggest that the bactericidal effect is concentration dependent (Close et al. However, in an in vitro pharmacodynamic model simulating kinetics with standard CoT doses, CoT was initially bactericidal for 8 hours, but over 24 hours of regrowth occurred, giving out a bacteriostatic pattern to 72 hours (LaPlante et al. Approximately 10% of the excreted drug is in the form of metabolites with little or no antibacterial activity. Urinary concentrations of about 100 g/ml after the usual oral dose are approximately 100fold higher than those attained in serum and remain high for about 24 hours. Drug interactions Most of the studies of drug interactions have focused on CoT, especially because of its role in the treatment and prophylaxis of P. The serum half-life of phenytoin may be increased in the presence of CoT (Wilcox, 1981). Numerous reports since have documented this troublesome interaction (Wilcox, 1981; Gillman and Sandyk, 1985). However, serious adverse interactions as a result of this inhibition have not been reported. In patients without biliary obstruction, concentrations of the drug in bile are slightly higher than those in the serum 4 hours after a dose, but these fall to levels lower than in the serum 24 hours after a dose (Rieder, 1973). One of the most important interactions of CoThis with warfarin (vitamin K antagonist) (Hale and Lesar, 2014; Lane et al. It is a particularly common problem and can increase international normalized ratios out of the therapeutic range in up to two thirds of patients and result in dangerous levels of anticoagulation in one third (Glasheen et al. The reaction has been recognized for many years (Barnett and Hancock, 1975; Tilstone et al. When CoThis used in patients receiving methotrexate, there is a possibility that the effects of methotrexate will be enhanced, particularly in terms of increased blood dyscrasias. Apart from these effects, there do not appear to be any major pharmacokinetic interactions between CoT and methotrexate (Beach et al. Most often the enhancement of methotrexate toxicity is noted in the context of methotrexate use for rheumatological disease, when bone marrow suppression from methotrexate must be avoided rather than expected (Thomas and Gutterman, 1986; Ng et al. Recent case-control studies have not demonstrated a relationship between concurrent CoT use and highdose methotrexate (> 1 g per square meter) in oncology patients (Chan and Rajakumar, 2014). However, in a systematic review of patients with rheumatological conditions, CoT with methotrexate was associated with higher rates of cytopenia (Bourre-Tessier and Haraoui, 2010).
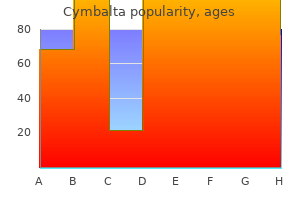
Discount 30 mg cymbalta amex
Unmetabolized drugs retain their lipid solubility and may undergo extensive tubular reabsorption anxiety symptoms without feeling anxious cheap 20 mg cymbalta visa, whereas metabolism increases the polarity of the drug molecules, making them more hydrophilic and encouraging renal elimination. Sulfonamide clearance is increased in the presence of alkaline urine (Williams et al. Furthermore, all the sulfonamides and their acetylated conjugates are more soluble in alkaline urine with the exception of sulfamethizole, which is highly soluble even in acid urine (Peddie and Little, 1979). The solubility in urine of the various sulfonamides and their conjugates varies considerably. Sulfadimidine and its acetylated form are very soluble compared with other sulfonamides, such as sulfadiazine. Homozygotes for the wild-type allele are rapid acetylators, compound heterozygotes for the wild-type and mutant alleles are intermediate acetylators, and homozygotes for the mutant alleles are slow acetylators (Tanigawara et al. Slow acetylator status has been associated with increased rates of adverse reactions to arylamine antibiotics such as sulfamethoxazole (Wolkenstein et al. Some of the sulfonamides are converted to inactive metabolites in the liver by glucuronidation. This process is particularly marked with sulfadimethoxine, 80% of which is excreted as a very soluble glucuronide in the urine (Busch and Lane, 1967). Sulfamethoxazole undergoes N4-hydroxylation in humans predominantly via the cytochrome P450-2C9 subfamily (Cribb et al. A sequence of reactions forms reactive metabolites responsible for mediating hypersensitivity reactions. These patients had greatly increased serum tolbutamide levels, and it appeared that sulfaphenazole interfered with oxidation and excretion of tolbutamide. The metabolism by liver microsomal enzymes of other drugs, such as diphenylhydantoin (phenytoin) and warfarin (and tolbutamide), is inhibited by usual therapeutic doses of sulfaphenazole, sulfadiazine, and sulfamethizole. Cotrimoxazole also increases the diphenylhydantoin half-life in patients by decreasing its metabolism (Hansen et al. In contrast, sulfadimethoxine, sulfamethoxypyridazine, and sulfamethoxydiazine do not affect phenytoin metabolism. Reduction in warfarin metabolism associated with concomitant sulfonamide administration may result in potentiation of warfarin-induced anticoagulation (Barnett and Hancock, 1975; Hassall et al. This causes an increase in the plasma concentration of levorotatory warfarin and a decrease in dextrorotatory warfarin. Because levorotatory warfarin is intrinsically more potent than dextrorotatory warfarin, there is a net increase in warfarin activity. Similar interactions occur between warfarin and metronidazole (see Chapter 99, Metronidazole). Potentiation of warfarin-induced hypoprothrombinemia has also been reported with sulfasalazine (Hall and Rindone, 2011). Alcohol can decrease the apparent half-life of sulfadimidine by increasing the amount of its acetylated form in blood and urine. Experience with five cardiac transplant Only small amounts of the sulfonamides are excreted via the biliary tract, and they are not concentrated in bile. Although the products of acetylation have no antibacterial activity, they retain the toxic potential of the parent sulfonamide. Active acetylators of sulfadimidine also rapidly inactivate isoniazid and vice versa, because a similar acetyltransferase enzyme is involved in the processing of both drugs. Patients who, after a test dose of sulfadimidine, have a proportion of acetylated drug less than 25% in the serum or less than 70% in the urine may be considered to be slow sulfonamide acetylators and will also usually be slow isoniazid inactivators (Rao et al. The remainder of absorbed sulfasalazine is excreted unchanged in the bile and, together with the nonabsorbed portion of the drug, reaches the distal small intestine and colon. However, some is also found in the acetylated form owing to gut wall and systemic acetylation. Furthermore, concomitant administration of cotrimoxazole and cyclosporine has been associated with an increased incidence of nephrotoxicity, independent of the levels of either drug (Sands and Brown, 1989). Sulfapyridine may displace methotrexate from protein binding sites and increase the risk of methotrexate myelotoxicity. Blood folate concentrations were higher in those children with late treatment failure. Fosamprenavir shares a sulfonylarylamine structure with sulfamethoxazole and darunavir (Phillips and Mallal, 2007). There is limited information on cross-reactivity between these drugs, and patients with sulfa or sulfonamide antibiotic hypersensitivity were not excluded from darunavir clinical trials. Magnetic resonance imaging demonstrated diffuse white matter abnormalities that resolved within months. Although malaria itself can be associated with neurological manifestations, the symptoms occurred within 50 minutes of the first dose. Sulfasalazine has been associated with a case report of centralvariant posterior reversible encephalopathy, with resolution of symptoms within 5 days of cessation of sulfasalazine and normalization of imaging at 30 days (Ocek et al. There is also a case report of facial palsy associated with sulfasalazine use (Magnus et al. Drug fever Drug fever is rare with commonly used short-acting sulfonamides, such as sulfadimidine and sulfafurazole, although it was frequent with the earlier sulfonamides. Drug fever has been occasionally observed with the long-acting sulfonamide, sulfamethoxypyridazine (Grieble and Jackson, 1958). Gastrointestinal side effects Nausea, vomiting, and diarrhea were common with earlier compounds such as sulfapyridine but are uncommon with the newer sulfonamides. Hypersensitivity reactions the mechanisms of idiosyncratic sulfonamide toxicity have not been clearly defined, although a number of authors have demonstrated an association between sulfonamide toxicity and slow acetylator status (with subsequent reduced ability to detoxify oxidative metabolites) (Shear et al. In an in vitro assay, lymphocytes from 6 patients with a history of severe reactions to sulfonamides were compared with those of 20 controls. The lymphocytes of the sulfa-allergic patients demonstrated increased toxicity from sulfonamide metabolites but not from the drugs themselves (Shear et al. Thus, inherited differences in the rate of toxic metabolite production and detoxification, and the rate of acetylation of the parent drug, may contribute to hypersensitivity. The most common types of rashes are maculopapular or urticarial, but erythema nodosum, 6b. Neurotoxicity Headache and dizziness were commonly reported with the older sulfonamides but are rare with the newer compounds. In the prepenicillin era, toxic psychoses due to sulfonamides were well described (Little, 1942). Other disturbances of the nervous system such as drowsiness, fatigue, insomnia, nightmares, confusion, depression, vertigo, ataxia, and peripheral neuritis have been reported (Weinstein et al. Neurotoxicity, manifested by agitation, confusion, hallucinations, and seizures, has also been associated with generalized hypersensitivity reactions in a couple of cases (Smith et al. The risk of adverse cutaneous reactions to sulfadoxine is 40-fold higher when it is taken in regular doses for prophylaxis compared with single-dose treatment (Gimnig et al. Adverse reactions reported during long-term antibiotic therapy with sulfonamides for recurrent urinary tract infections in children occurred in 10. None of these were serious or life-threatening, and they were reversible on drug cessation. The rate was higher in children younger than 2 years of age, and fewer than 10% of antibiotic courses were discontinued in children younger than 2 years of age. In its most extensive form, this syndrome consists of erythema multiforme and ulceration of the mucus membranes of the eyes, mouth, and urethra, which can be very severe and sometimes fatal.
Java citronella (Citronella Oil). Cymbalta.
- What other names is Citronella Oil known by?
- How does Citronella Oil work?
- What is Citronella Oil?
- Are there safety concerns?
- Dosing considerations for Citronella Oil.
- Preventing mosquito bites when applied to the skin. Citronella oil is an ingredient in some commercial mosquito repellents. It seems to prevent mosquito bites for a very short amount of time. Other mosquito repellents, such as those containing DEET, are usually preferred because these repellents last much longer.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96619
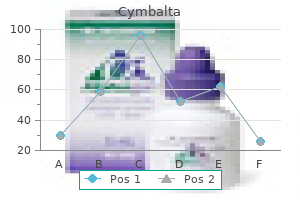
Buy cymbalta with visa
Sequential anxiety 25 mg zoloft discount 20mg cymbalta with visa, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: A prospective randomized study. A meta-analysis of the efficacy of albendazole compared with tinidazole as treatments for Giardia infections in children. Comparison of chlorioquine, albendazole and tinidazole in the treatment of children with giardiasis. Susceptibilities to different antibiotics of Helicobacter pylori strains isolated from patients at the pediatric medical center of Tehran, Iran. Sequential therapy or standard triple therapy for Helicobacter pylori infection: An updated systematic review. Improved efficacy of 10-day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Single-dose treatment of giardiasis in children: a comparison of tinidazole and metronidazole. Antimicrobial prophylaxis in upper gastrointestinal, biliary, stomach and oesophageal surgery. Randomized controlled comparison of nitroimidazoles for the eradication of Helicobacter pylori and relief of ulcer-associated and non-ulcer dyspepsia. Therapy for Helicobacter pylori infection can be improved: sequential therapy and beyond. Successful response of metronidazoleresistant trichomonal vaginitis to tinidazole. Comparison of ornidazole and tinidazole in single-dose treatment of trichomoniasis in women. Low dose, short-term triple therapy for cure of Helicobacter pylori infection and healing of peptic ulcers. Rapid and selective high-performance liquid chromatographic method for the determination of metronidazole and its active metabolite in human plasma, saliva and gastric juice. Comparative activity of metronidazole and tinidazole against Clostridium difficile and Peptostreptococcus anaerobius. Metronidazole, tinidazole, ornidazole and anaerobic infections of the middle ear, maxillary sinus and central nervous system. Comparative evaluation of the 2-methyl-5nitroimidazole compounds dimetridazole, metronidazole, secnidazole, ornidazole, tinidazole, carnidazole, and panidazole against Bacteroides fragilis and other bacteria of the Bacteroides fragilis group. In vitro susceptibility of Giardia lamblia trophozoites to metronidazole and tinidazole. An evaluation of tinidazole as single-dose therapy for the treatment of Trichomonas vaginalis. Efficacy and tolerability of a combination of ofloxacin and tinidazole in the management of infectious diabetic foot ulcer. Metronidazole and tinidazole in a single large dose for treating urogenital infections with Trichomonas vaginalis in men. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Guar gum as a carrier for colon specific delivery; influence of metronidazole and tinidazole on in vitro release of albendazole from guar gum matrix tablets. Comparative evaluation of tinidazole and metronidazole in the treatment of amoebic liver abscess. Evaluation of treatment regimens to cure Helicobacter pylori infection-a meta-analysis. Amebic abscess of the spleen complicated by metronidazole-induced neurotoxicity: Case report. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: A randomized controlled trial. Helicobacter pylori infection in a pediatric population: in vitro susceptibilities to omeprazole and eight antimicrobial agents. Triangular test design to evaluate tinidazole in the prevention of Plasmodium vivax relapse. Current concepts in the management of Helicobacter pylori infection-the Maastricht 2-2000 Consensus Report. Tinidazole: from protozoa to Helicobacter pylori-the past, present and future of a nitroimidazole with peculiarities. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Helicobacter pylori isolates from proximal and distal stomach of patients never treated and already treated show genetic variability and discordant antibiotic resistance. Comparison between 10- and 14-day hybrid regimens for Helicobacter pylori eradication: A randomized clinical trial. Efficacy of the combination of 2 g oral tinidazole and acidic buffering vaginal gel in comparison with vaginal clindamycin alone in bacterial vaginosis: a randomized, investigator-blinded, controlled trial. Antihelminthic efficacy of tinidazole against the progression of Toxocara canis larvae to the brain in mice. Use of prophylactic tinidazole to avoid the serious infections associated with total abdominal hysterectomy. The effectiveness of omeprazole, clarithromycin and tinidazole in eradication of Helicobacter pylori in a community screen and treat programme. Comparison of 2 g single dose of metronidazole, nimorazole and tinidazole in the treatment of vaginitis associated with Gardnerella vaginalis. One-week triple therapy with omeprazole, clarithromycin, and nitroimidazole for Helicobacter pylori infection in children and adolescents. Effects of metronidazole and tinidazole ointments on models for inflammatory dermatitis in mice. Microbiological properties of tinidazole: spectrum, activity and ecological considerations. Eighteen month follow up of Helicobacter pylori positive children treated with amoxycillin and tinidazole. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: A systematic review of randomized controlled trials. The syndromic management of vaginal discharge using single-dose treatments: a randomized controlled trial in West Africa. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. In vitro susceptibility testing of nonsporing anaerobes to ten antimicrobial agents. A comparison of the in vitro activity of metronidazole, tinidazole and nimorazole against Gram-negative anaerobic bacilli. The susceptibility of Helico bacter pylori to 12 antimicrobial agents, omeprazole and bismuth salts. Effect of triple therapy or amoxycillin plus omeprazole or amoxycillin plus tinidazole plus omeprazole on duodenal ulcer healing, eradication of Helicobacter pylori, and prevention of ulcer relapse over a 1-year follow-up period: a prospective, randomized, controlled study. Evaluation of invitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: A prospective randomized trial. Sensitivity of Gardnerella vaginalis to metabolites of metronidazole and tinidazole. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Anaphylaxis and hypersensitivity syndrome reactions in increasing severity following repeated exposure to tinidazole. Short course of single daily dosage treatment with tinidazole and metronidazole in intestinal amoebiasis: a comparative study. Outcomes of transrectal ultrasound scan of the prostate with sector biopsies for 323 New Zealand men with suspicion of prostate cancer. A case of high-level metronidazoleresistant trichomoniasis in pregnancy successfully treated. Fixed drug eruption due to metronidazole and tinidazole without cross-sensitivity to secnidazole.
Buy cymbalta 20 mg mastercard
Emergence of ciprofloxacin resistance was noted in 62% of patients who did not show any bacteriologic response (Fang et al anxiety 2 60 mg cymbalta free shipping. Fluoroquinolones, including ciprofloxacin (single dose), appear to be useful, given either i. Clinical uses of the drug 1915 Interstitial cystitis is a chronic condition of unknown etiology characterized by bladder pain, urinary urgency, and urinary frequency. An infectious etiology has not been clearly documented, but various organisms can be cultured from urine specimens, and some patients report symptomatic improvement in association with antibiotic use. In an open pilot study involving 50 women with this condition who received sequential therapy with 3 weeks each of doxycycline, erythromycin, metronidazole, clindamycin, amoxicillin, and ciprofloxacin, all in conjunction with rifampicin, there was no significant improvement in symptoms in comparison with placebo (Warren et al. Perhaps not surprisingly, there was a significant increase in adverse effects among patients receiving active agents. This study suggests that antibiotics do not have a role to play in this condition. Nevertheless, despite many clinical studies of fluoroquinolone efficacy in this condition, a comparison of clinical results remains difficult because of differences in study methodology, lack of a clear clinical definition of prostatitis, and variable use of diagnostic techniques, such as the standard four-specimen technique for localizing infection to the prostate (Meares and Stamey, 1968). Furthermore, many studies combine the treatment results for acute and chronic prostatitis rather than reporting them separately. Other fluoroquinolones may have similar efficacy, but good clinical studies are lacking. A recent Cochrane review of randomized controlled trials for chronic bacterial prostatitis found no significant differences in clinical or microbiological efficacy or rates of adverse reactions between different fluoroquinolones (Perletti et al. However, no conclusion was drawn on the most appropriate duration of treatment with fluoroquinolones for chronic prostatitis. Therapies are empirical and mostly untested and, although there has been no clear evidence to support an infective etiology, antibiotics are frequently prescribed. In one study involving 196 men diagnosed with this condition and a mean duration of symptoms of 6. Thus, long-term ciprofloxacin should not be recommended for this complex pain syndrome. Few studies have formally examined the efficacy of fluoroquinolones in the treatment of epididymitis and epididymo-orchitis, but the known antibacterial spectra and pharmacokinetics of fluoroquinolones suggest that they are a potentially appropriate therapeutic choice. Humphreys and Speller (1989) reported three patients with acute epididymo-orchitis caused by P. Bacterial gastroenteritis and enteric fever Fluoroquinolones have generally excellent in vitro activity against the majority of gastrointestinal bacterial pathogens, such as Salmonella spp. In a double blind randomized trial comparing a 5-day course of ciprofloxacin 500 mg twice daily with ampicillin 500 mg four times daily in 121 adults hospitalized for shigellosis in Bangladesh, Bennish et al. Overall, bacteriologic cure was more common in the ciprofloxacin-treated group (p < 0. Ciprofloxacin and azithromycin were compared in a randomized double blind trial in 70 men in Bangladesh with shigellosis. Other authors have also advocated the use of 1 g single dose ciprofloxacin for shigellosis (Williams and Richards, 1990; Bhattacharya et al. One or two dose regimens have particular appeal in the treatment of shigellosis in children, when the potential risks associated with fluoroquinolone use in this age group are considered by some authors to be outweighed by the potential benefits of effective therapy (Fontaine, 1989). In a study of 120 children with shigellosis, 5 days of ciprofloxacin was associated with a significantly superior bacteriologic cure rate than pivmecillinam, but the better clinical cure rate was not statistically significant (Salam et al. However, ciprofloxacin and other fluoroquinolones have been shown by many authors to be beneficial in this context (DuPont et al. Both the duration of diarrheal illness and fecal shedding in patients with Salmonella disease may be reduced with ciprofloxacin therapy (Hudson et al. However, the definition of cure in this study was more restrictive than that used by Pichler et al. Furthermore, this study explicitly excluded patients with severe disease or who were at risk of bacteremia, and deliberately chose patients with short clinical histories before study entry. Other studies, although not placebo-controlled, have suggested good clinical efficacy with ciprofloxacin 500 mg twice daily for 7 days for the treatment of outbreaks of multiresistant nonenteric S. Management of institutional outbreaks of Salmonella gastroenteritis, including the use of ciprofloxacin, has been reviewed by Lightfoot et al. However, emerging resistance to fluoroquinolones, including ciprofloxacin (see section 2b, Emerging resistance and cross-resistance), among Salmonella species now potentially threatens the efficacy of these agents, and treatment failures are being increasingly reported both for non-typhoid (Collard et al. Ciprofloxacin has also proven effective against nonenteric sites of salmonellosis, including empyema and neonatal brain abscesses due to S. In a randomized, nonblinded study of uncomplicated enteric fever infections (28 S. Clinical failure was noted in none of 20 ciprofloxacin-treated patients compared with 6 of 22 (27%) ceftriaxone-treated patients (five of these six patients had persistent fevers for > 7 days; p = 0. The mean duration of fever was 4 days for ciprofloxacin-treated patients compared with approximately 5 days for ceftriaxone-treated patients (p = 0. All isolates were susceptible to both agents, but seven of 12 strains examined in detail were resistant to ampicillin, chloramphenicol, and trimethoprim. In a randomized trial of 55 adults with enteric fever in Indonesia who underwent blood and bone marrow culture after treatment with either ciprofloxacin or chloramphenicol (all isolates were susceptible to these agents), ciprofloxacin was associated with a higher rate of elimination of S. However, for uncomplicated typhoid fever, oral azithromycin (see Chapter 62, Azithromycin) appeared to be equally as effective as ciprofloxacin in a randomized trial in Egypt in which all isolates were susceptible to both agents (Girgis et al. Other authors have described excellent rates of cure in open, noncomparative studies of either oral, or combination i. All patients requiring treatment for 10 days had prolonged fever (10 days) before presentation and commencement of therapy. This study suggests that ciprofloxacin 500 mg twice daily is adequate treatment for typhoid fever, but the duration of treatment may need to be individualized depending on the duration of pretreatment symptoms and time to defervescence. Comparisons of duration of fluoroquinolone therapy for typhoid fever were summarized in a recent Cochrane Database review (Effa et al. Statis- tically, no significant differences in outcomes or adverse events were observed with 2 days vs. In two recent literature reviews, it was noted that although fluoroquinolones are generally effective in the treatment of enteric fever, the increasing prevalence of isolates with reduced susceptibilities may need to be considered in empiric treatment regimens (Butler, 2011; Effa et al. As with non-typhoidal salmonellosis, ciprofloxacin has proven effective in the treatment of severe typhoid in children (Dawood et al. Children regained consciousness within a mean of 2 days, were afebrile within a mean of 3. One child who was severely malnourished died 24 hours after ciprofloxacin therapy was commenced. These results appear promising in comparison with the previous standard of chloramphenicol therapy in this setting-especially considering the increasing emergence of chloramphenicol resistance among S. Ciprofloxacin appears to be safe when given during pregnancy for multiresistant S. All pregnancies were carried to term and healthy babies with no congenital abnormalities were delivered. Manufacturer reports of 130 women, mostly with first trimester pregnancies, who accidentally received ciprofloxacin noted that none of these babies had any congenital abnormalities (Koul et al. Although other fluoroquinolones, such as pefloxacin and ofloxacin, also appear to be effective in typhoid fever (Hajji et al. However, the emergence of resistance to ciprofloxacin and other fluoroquinolones (see section 2b, Emerging resistance and cross-resistance) now poses a major therapeutic 1918 Ciprofloxacin concern, such that in these resistant cases, third-generation cephalosporins such as ceftriaxone (see Chapter 27, Ceftriaxone) are now often required for effective treatment of these strains (Bhan et al. Ciprofloxacin and norfloxacin are effective in eradicating both non-typhoidal Salmonella and S.
Buy cymbalta us
This may not apply to patients with acute liver disease and severe hepatic impairment (Peter et al anxiety symptoms unsteadiness purchase generic cymbalta from india. In vitro studies have suggested that fusidate sodium is very highly protein bound; one study showed that the bind ing was as high as 97. The in vitro activity of the drug is reduced 64fold in the presence of 50% serum (Barber and Waterworth, 1962). Despite this very high degree of serum protein binding, the drug is quite effective clinically. Recent studies on fusidic acid pharmacokinetics have given new insights into the complex relationship between dose and clearance of the drug (Bulitta et al. Accumulation of fusidic acid after multiple doses is higher than that predicted based on singledose data. The pharmacokinetics of fusidic acid is best described as a timedependent mixedorder absorption process, two dis position compartments, and a turnover process to describe the socalled autoinhibition of clearance. As a result of the autoinhibition of clearance, steady state can be achieved earlier with dosing regimens that con tain higher doses (after 8 days for 750 mg every 12 h, or after 1 day for 1500 mg every 12 h on day 1 followed by 600 mg every 12 h, respectively, vs. Because large initial doses autoinhibit the clearance of fusidic acid, this characteristic provides a basis for the administration of frontloaded dosing regimens of sodium fusidate, which allows for effective concentrations to be achieved early in ther apy (Bulitta et al. Concentrations then gradually fell, and detectable amounts were still present after 24 hours (Godtfredsen et al. The serum elimina tion halflife ranges from 10 to 16 hours after oral or intrave nous administration of 500 mg of sodium fusidate (see Table 80. After 96 hours of such a regimen, a mean serum level of 71 mg/l was obtained, but some indi viduals showed serum levels just over 100 mg/l (Godtfredsen et al. After a single intra venous infusion of 500 mg of sodium fusidate over 2 hours, the peak serum level just after the infusion was 52 mg/l; if this dose is given intravenously every 8 hours for 3 days, the peak serum level after the last infusion is approximately 123 Sodium fusidate can be administered to elderly patients in the same dosages used for both oral and intravenous admin istration in adults (see earlier). Bioavailability After oral administration, the bioavailability of sodium fusi date is 69% for a capsule formulation and 46% for a fusidic acid suspension (Wise et al. Food was shown not to affect total drug absorption, although it did significantly 1412 Fusidate Sodium Table 80. Pharmacokinetic data for fusidic acid after various types of tablets (oral) or intravenous dosing in adults. Thus there is some accumulation of the drug in body with repeated infusions (Taburet et al. At dos ages of 750 mg or higher every 12 hours, inhibition of clear ance was evident both after single doses and at steady state. At these higher doses, significant accumulation of fusidic acid therefore occurs irrespective of schedule or administra tion regimen (Bulitta et al. Serum elimination halflife generally ranged between 14 and 29 hours, with the longer halflives after the 11th doses (Bulitta et al. It has been demonstrated in samples of subcutaneous fat, kidney, muscle, and prostate taken from patients undergoing surgery a few hours after its administration. Fusidate sodium is effective even in the presence of large collections of pus, possibly because of its ability to pen etrate well into purulent collections (Crosbie, 1963). Fusidic acid penetration in skin blister fluid was studied in healthy humans after intake of 250 mg or 500 mg twice a day for 6 days, by Vaillant et al. It must be noted, however, that these are total concentrations and not free, unbound concentrations. The penetration of topical fusidic acid through intact skin from cadavers has been measured to be around 2% (Vickers, 1969). High concentrations of fusidic acid have been mea sured in the conjunctival fluid of rabbit eyes after topical application of 1% sodium fusidate eye drops in the lower eye lid. The penetration of a 1% fusidic acid suspension through the cor nea before cataract surgery resulted in aqueous humor con centrations of 0. High fusidic acid concentrations were found in synovial fluid in patients with rheumatologic disorders (Somekh et al. Clinically important pharmacokinetic and pharmacodynamic features Some investigators have reported that this drug is mainly bacteriostatic (Hilson, 1962), whereas others have found it to be bactericidal (Newman et al. These differing results seem to depend on such factors as the concentration of the drug used, the inoculum size, and the bacterial species stud ied; for example, in one study fusidic acid was more bacteri cidal against an S. With use of the hollowfiber model, delay in development of resistance was used as the effect parameter by the same investigators in the study of optimal dosing regimens (Okusanya et al. Treatment outcome of fusidic acid or comparator antibiotics for treatment of skin and soft tissue infections in adults according to etiology or total clinical cure/improvement (Number of patients cured/Number of patients or infections treated). Clinical or bacteriologic parameter Staphylococcus aureus Beta-hemolytic streptococci Clinical cure/improvement S. The standard pharmacodynamic/ pharmacokinetic relationship studies of fusidic acid in experi mental rodent models have been cumbersome because fusidic acid concentrations are rather low in rodents such as mice. Still, there are solid clinical data on the effect of sodium fusidate adminis tered orally even in doses of 250 mg twice a day against beta hemolytic streptococci causing tissue or soft tissue infections (Table 80. Very little active fusidate sodium is excreted by the kidneys, and only about 1% of the administered dose can be recovered from the urine. A fraction of the administered dose can be recovered in active form from the feces; some of this may be nonabsorbed drug, and some presumably a result of biliary excretion. Fecal excretion of fusidic acid is minimal (Reeves, 1987), but oral sodium fusidate is effective as treatment for C. This may have occurred as a result of either competition for metabolism in the liver or displacement because of the high serum proteinbinding of fusidic acid. Three case reports of rhabdomyolysis secondary to concom itant treatment with simvastatin and fusidic acid suggested that fusidic acid somehow competes for metabolism with the cyto chrome P3A4 enzyme system, which is known to take part in the metabolism of simvastatin (Yuen and McGarity, 2003). Excretion A major proportion of the drug is converted to metabolites in the liver, some of which have weak antibiotic activity (Reeves, 1987). When administered orally, only mild upper gastroin testinal discomfort and diarrhea have been noted. Investigations have failed to show any evidence of renal or hemopoietic tox icity. There is a risk of thrombophlebitis after intravenous administration into a peripheral vein for more than 24 hours (Iwarson et al. No severe allergic reactions have been observed, but occasional mild rashes have been reported. In chronic infections, fusidate sodium has been given for several months without obvious toxic effects (Crosbie, 1963; Dodson, 1963). It is not known whether fusidate sodium accumulates in the presence of liver disease, and therefore it should be used cautiously in patients with impaired liver function. In a group of patients with staphylococcal septicemia treated with intravenous fusi date sodium or other antibiotics, the rates of development of jaundice in these two groups were 34% and 2%, respectively. Of the jaundiced patients, 48% received the old fusidate sodium intravenously compared with 13% by mouth; jaun dice appeared within 48 hours of commencing this drug in 93%; it was associated with deepening of jaundice in 68% of those with preexisting jaundice (Humble et al. When fusidate sodium was stopped, serum bilirubin values fell to normal within 4 days in those who were anicteric before treatment. In 6 of 32 patients receiving the drug intrave nously, liver function test results suggested a cholestatic pic ture; in the remainder, the mechanism of production of jaundice was unknown (Humble et al. The new intravenous sodium fusidate prepa ration can also cause jaundice; whether it does so less fre quently is not yet clear. Some 6% of patients receiving the new filmcoated sodium fusidate tablets also have developed jaundice (Eykyn, 1990; Portier, 1990). Because of the steroid structure of fusidate sodium, it was thought that this drug may possibly have some metabolic effects unrelated to its antibacterial activity. Wynn (1965) showed that no significant metabolic changes were associ ated with fusidate sodium administration. It had a mild pro tein catabolic effect, it lowered urinary calcium excretion, and it also caused mild temporary impairment of bromsulphthalein excretion by the liver. It is conceivable that the latter finding may have some relation to the ability of the drug to impair liver func tion.
Quality 60mg cymbalta
As a result anxiety symptoms wikipedia purchase cheapest cymbalta and cymbalta, indications for the use of this combination were altered, and Fansidar is no longer recommended for prophylaxis against malaria or pneumocystis pneumonia (see Chapter 93, Pyrimethamine). Hypersensitivity associated with reactivation of human herpesvirus 6 and induction of antiphospholipid antibodies has been reported with sulfasalazine use (Tung et al. Contrary to these data, more recent studies have suggested that there may not be an association between slow acetylator genotype and hypersensitivity reactions (Pirmohamed et al. Glutathione is important in protecting cells from the effects of sulfamethoxazole metabolites by preventing the oxidation of the hydroxylamine to the more toxic nitroso metabolite (Lin et al. Adverse reactions and toxicity 1589 success (Holdsworth, 1981; Taffet and Das, 1982; Finegold, 1985; Hughes et al. They used a solution containing either 4 mg/ml of sulfamethoxazole or 5 mg/ml of sulfadiazine, of which 1 ml was given every 6 hours for four doses; then, if no reaction was observed, the dose was doubled on the same every-6-hours schedule every 24 hours until the desired dose was reached (sulfamethoxazole, 1. About 2% of patients receiving sulfasalazine therapy for inflammatory bowel disease (see section 7m, Inflammatory bowel disease) develop symptoms that appear to be allergic (Purdy et al. This is distinct from the nonallergic toxicity of sulfasalazine, which is related to the serum sulfapyridine level. Allergic manifestations usually consist of a skin rash (urticaria, macular or diffuse erythema) with or without fever and are not associated with the acetylator phenotype of the patient. Desensitization should not be attempted in patients with serious reactions to sulfasalazine, such as agranulocytosis, toxic epidermal necrolysis, or fibrosing alveolitis. A generalized hypersensitivity reaction associated with fever, arthralgia, lymphadenopathy, and hepatitis can occur with sulfasalazine. Hypersensitivity manifestations after topical sulfonamide therapy are common, and this form of treatment is now used only rarely. Topical mafenide has been associated with allergic contact dermatitis (Firoz et al. There have been several case reports of mafenide acetate hypersensitivity mimicking acute suppurative chondritis (Pickus et al. The hypersensitivity reaction lacks the fever, fluctuance, and pain usually seen in acute suppurative chondritis, and symptoms resolve within days after cessation of mafenide with or without concomitant corticosteroid therapy. Toxic epidermal necrolysis has been associated with the use of topical sulfacetamide eye drops (Byrom et al. There is usually cross-allergy between all the sulfonamides, and sulfonamide-allergic patients may potentially be allergic to other drugs of similar structure such as furosemide, hydrochlorothiazide, and glipizide (and vice versa) (Sullivan, 1991). It is unwise to administer any sulfonamide to a patient with a previous history of allergy to one of these drugs. The immunological determinant of type I (immediate hypersensitivity) responses to sulfonamide antibiotics is the N1 heterocyclic ring, which nonantibiotic sulfonamides do not contain. Formation of these metabolites is a stereospecific process that occurs at the N4 amino nitrogen of the sulfonamide antibiotics, a structure also not found on any nonantibiotic sulfonamide drugs. The stereospecificity of these reactions implies that cross-reactivity with nonantibiotic sulfonamide-containing drugs is highly unlikely (Brackett et al. T cell recognition of unmetabolized, nonhaptenated parent sulfonamide antibiotic may occur in a small subset of hypersensitive patients. An association was found between hypersensitivity after treatment with sulfonamide antibiotics and subsequent sulfonamide nonantibiotic use, but there was also an association with subsequent penicillin use. This indicates a possible predisposition to allergic reactions rather than cross-reactivity between sulfonamide-based drugs. Hemstreet and Page (2006) studied potential cross-reactivity between sulfonamide antibiotics and nonantibiotics. Of those patients with positive controlled oral challenge and patch testing, none developed positive results with sulfonamide nonantibiotics. One exception appears to be cross-reactivity between sulfasalazine and sulfamethoxazole, for which lymphocyte transformation tests from patients with known hypersensitivity to sulfasalazine were positive for both sulfapyridine and sulfamethoxazole (Zawodniak et al. A more recent review of the literature including manufacturer-provided data did not find any convincing evidence of broad cross-reactivity between antibacterial sulfonamide compounds and nonantibacterial sulfonamides (Wulf and Matuszewski, 2013). Hematological toxicity the most common hematological toxicity is acute agranulocytosis, although aplastic anemia, megaloblastic anemia, and thrombocytopenia have also been described. Agranulocytosis 1590 Sulfonamides was reasonably common with the older sulfonamides, such as sulfapyridine, but is rare with currently used drugs. In a survey in Sweden between 1973 and 1978, sulfonamides were one of the most frequent causes of druginduced leukopenia (Leading article, 1983b). Usually treatment can be continued if the total leukocyte count is greater than 3000 per cm2. The sulfapyridine part of sulfasalazine is absorbed, and acute agranulocytosis due to this drug has been reported in two patients, both of whom died with septicemia (Thirkettle et al. The average duration between sulfasalazine exposure and onset of acute agranulocytosis was 42 days, with normalization of neutrophil count occurring within a mean of 11 days (Andersohn et al. Agranulocytosis attributed to sulfasalazine was also reported in a case-control surveillance study of drug-induced agranulocytosis (Huber et al. Agranulocytosis associated with the use of dapsone in the treatment of dermatitis herpetiformis has been estimated to occur with an increased relative risk of 50-fold, or a total risk of one case per 3000 patient-years of exposure to dapsone (median treatment dose of 100 mg of dapsone daily) (Hornsten et al. Other hematological complications, such as hemolytic anemia, alterations in erythrocyte morphology, erythroid hypoplasia, and pancytopenia, have been described with sulfasalazine. One patient had agranulocytosis, erythroid hypoplasia, and bone marrow plasmacytosis due to sulfasalazine (Wheelan et al. A patient treated with sulfasalazine for ulcerative colitis on a background of hemodialysis developed diarrhea and leukopenia (Teshima et al. This may have led to a rise in sulfapyridine concentration, and coexisting reduction in protein binding due to end-stage renal failure may have potentiated this effect because sulfasalazine is extensively protein bound. Fatal aplastic anemia has been attributed to sulfonamides, but less commonly than to chloramphenicol. Aplastic anemia and two cases of pure red cell aplasia have been described in association with sulfasalazine therapy (Dunn and Kerr, 1981; Anttila et al. Acute hemolytic anemia is another rare complication and is sometimes due to prior sensitization to sulfonamides (Weinstein et al. This type of anemia may also occur in fetuses or premature infants whose red cells are normally deficient in glucose6-phosphate dehydrogenase. Megaloblastic anemia responding to therapy with folic acid has been described in patients with ulcerative colitis who were being treated with sulfasalazine (Schneider and Beeley, 1977; Kane and Boots, 1977). Folate deficiency may occur in patients with inflammatory bowel disease, and some studies have indicated that sulfasalazine therapy further impairs folate absorption (Halsted et al. Other investigations suggest that in patients with inactive chronic colitis taking an optimum maintenance dose of 2 g or less of sulfasalazine daily, folate deficiency would be rare. However, subclinical tissue depletion could occur with higher doses, particularly if other factors, such as deficient dietary intake, severe bowel inflammation, pregnancy, associated hemolysis, and small bowel disease or resection, are present, which increase the likelihood of folate deficiency (Longstreth and Green, 1983). Thrombocytopenia alone is a rare complication of sulfonamide therapy (Weinstein et al. Cyanosis due to the formation of either methemoglobin or sulfhemoglobin was fairly common with the earlier sulfonamides but is now rare with the currently used compounds. Sulfanilamide is converted to intermediate forms with direct oxidizing potential on hemoglobin.
Order discount cymbalta on line
In Indonesia anxiety fever discount cymbalta 40mg amex, malaria treatment is often administered without microscopic confirmation of P. Accumulated single-base changes within these genes can increase drug resistance by several orders of magnitude. Mutations were more frequent in those with pneumonia (40%) compared with those colonized (22%); however, mutations were also detected in patients without previous exposure to sulfa drugs, suggesting local transmission of drug-resistant strains from colonized individuals. It also inhibits cyclo-oxygenase- and lipoxygenase-dependent pathways (Elder et al. Sulfasalazine and sulfapyridine also inhibit endothelial cell chemotaxis and proliferation (Volin et al. Recent data suggest that sulfaphenazole attenuated cardiac reactive oxygen species levels and ischemia-reperfusion injury in rats by eliminating reactive oxygen species and inhibiting cytochrome P450 activity (Ishihara et al. Sulfasalazine and its metabolites also inhibit platelet function (MacMullan et al. Nearly all the sulfonamides are available as 500-mg tablets; some are also available as 250-mg or 1-g tablets or as a pediatric preparation. Parenteral sulfadiazine and sulfisoxazole diolamine are available in preparations of 250 and 400 mg/ml, respectively. However, these analogues probably contribute very little to the activity of sulfonamide. Resistance of malaria parasites to sulfonamides and other antifolate antimalarial drugs has been well studied, and the genetic basis for resistance is relatively well characterized (see earlier). Malaria parasites predominantly synthesize active folate cofactors de novo and utilize exogenous intact folate precursors in various forms (Milhous et al. Carbonic anhydrase is now being recognized as a new target of action for sulfonamides. A novel compound 4-(3,4-dichlorophenyl ureido-ethyl)benzenesulfonamide has the most potent activity against P. More research into the role of these agents as antimalarial agents is in progress. The immunomodulatory and anti-inflammatory action of sulfasalazine has several mechanisms. The adult dosage (including children older than 12 years) of sulfamethizole is 1 g three times daily. An entericcoated tablet is available for patients who develop gastrointestinal intolerance. For severe infections in children aged up to 6 years of age (20 kg of body weight), 40 mg/kg can be given once and then 20 mg/kg daily. However, owing to potential concerns about teratogenicity (see section 6, Adverse reactions and toxicity) and potential toxicity to the neonate, they should generally be avoided during pregnancy, but especially near term. Using computer modeling, these authors suggested that giving three daily sulfadoxine doses of 1. Clinical studies with this dose have not been performed, and the authors raised concern about patient compliance with this higher dose (see section 5a, Bioavailability). Sulfonamides are excreted in breast milk; hence caution is required, especially if the infant is premature, ill, jaundiced, or stressed, owing to the risk of bilirubin displacement and kernicterus (see section 5b, Drug distribution, and section 6, Adverse reaction and toxicity). Newborn infants and children Sulfonamides are contraindicated in neonates (see section 6, Adverse reactions and toxicity). In children older than 2 months, an initial dose of 75 mg/kg of body weight may be followed by a daily dosage of 150 mg/kg, given in four to six equally divided doses. The dosage of sulfamethizole for children aged greater than 2 months up to 12 years is 5 mg/kg three times daily. The recommended dosage for children is, therefore, proportionally greater than for adults (Follath, 1979). Sulfafurazole is given in a dose of 75 mg/kg initially, followed by 150 mg/ kg daily in divided doses to a maximum of 6 g daily. Pharmacokinetics and pharmacodynamics 1583 different renal mechanisms and at different rates (Gilbert, 2006). Fischer (1972) observed the sulfadimidine clearance in uremic patients to be significantly lower than in patients with normal renal function. Furthermore, urine concentrations of free sulfonamides were low in uremic patients. These authors concluded that sulfonamides are unlikely to be useful for treatment of urinary tract infections in patients with serum creatinine levels exceeding 5 mg/100 ml (0. Sulfonamides such as sulfafurazole or sulfamethoxazole, which have lower inhibitory concentrations for common urinary pathogens and are less readily conjugated, may be preferable to sulfadimidine for the treatment of urinary tract infections in uremic patients (Adam et al. Pharmacokinetic studies with sulfadiazine indicate that about 60% of the drug is excreted unchanged via the kidneys and that the remainder is eliminated extrarenally. There are no useful data regarding sulfadiazine use in hemodialysis or peritoneal dialysis. In studies of renal transplant recipients, renal elimination of sulfisoxazole was decreased and correlated with creatinine clearance (Shermantine et al. For treatment of systemic infections, sulfonamides can probably be safely used in most patients with renal disease, but regular estimations of the serum concentrations of both free and acetylated sulfonamide seem advisable. The various sulfonamides are handled by the kidney in different ways (see section 5b, Drug distribution), and the rate of acetylation of sulfonamides in the body varies in individual patients. For these reasons, in some uremic patients it may be impossible to select a dose that will give adequate serum levels of the free active drug yet not lead to accumulation of toxic levels of acetylated compounds. The relationship between toxicity and serum levels in the case of the sulfonamides is not well defined, but some patients with a total serum level of greater than 100 mg/ml often show toxic effects (Adam and Dawborn, 1970). There is an increased risk of adverse drug reactions in the elderly, including cutaneous eruptions and bone marrow suppression. This is accentuated by the presence of coexisting renal and/or hepatic impairment. Bioavailability All sulfonamides, except sulfaguanidine and the other two compounds of that group (see Table 91. After absorption, these drugs are partly conjugated with acetate in the liver, and the proportion conjugated varies with different sulfonamides. Sulfonamide conjugates are inactive therapeutically, and the serum level of the free active (unconjugated) drug is important.