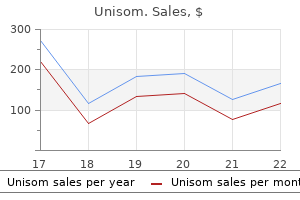
Discount 25mg unisom fast delivery
Studies thus far indicate that in sarcomas sleep aid otc list cheap generic unisom uk, the majority of genes fused by translocations encode transcription factors. Moreover, other chromosomal rearrangements leading to ret activation were recently described, especially in children of the Chernobyl-contaminated areas. Furthermore, although functionally different, the various activating genes share the following three properties: 1. They display domains demonstrated or predicted to be able to form dimers or multimers. An example is Akt, three serine-threonine kinases that represent major effectors mediating survival signal. Generally, Akt proteins possess six sites of phosphorylation: Ser124 and Thr450 are basally phosphorylated, Tyr315 and Tyr316 depend on Src, Thr308 represents the major site of regulation and is phosphorylated by 3-phosphoinositides-dependent protein kinase 1, Ser473 is only required for maximal Akt activity but the mechanism by which it is phosphorylated remains controversial. Once activated, Akt dissociates from the plasma membrane and translocates to both the cytoplasm and the nucleus. Akt determines cell survival also by virtue of its involvement in cell-cycle progression. Overall, 77 mutations were identified, in aggregate, affecting 26% of colorectal tumors analyzed. The great majority of the mutations would result in proteins devoid of phosphatase catalytic activity. Statistical analysis of the age incidence of human solid tumors indicates that five or six independent mutational events may contribute to tumor formation. In human leukemias, only three or four mutational events may be necessary, presumably involving different genes. The study of chemical carcinogenesis in animals provides a foundation for our understanding of the multistep nature of cancer. Cancer develops exclusively when the exposure to promoter follows the exposure to carcinogen (mutagen. For papillomas to appear on the skin, however, growth of mutated cells must be continuously stimulated by a promoting agent. Additional unidentified genetic changes must then occur for papillomas to progress to carcinoma. Although a single oncogene is sufficient to cause tumor formation, transformation by a single oncogene is not usually seen in experimental models of cancer. On the contrary, different oncogenes frequently cooperate in producing the neoplastic phenotype. For example, studies have shown cooperation between the nuclear myc protein and the cytoplasmic-membrane-associated ras protein in the transformation of rat embryo fibroblasts. The initiation and progression of human neoplasia involve the activation of oncogenes and the inactivation or loss of tumor-suppressor genes. The mechanisms of oncogene activation and the time course of events, however, vary among different types of tumors. In hematologic malignancies, soft-tissue sarcomas, and the papillary type of thyroid carcinomas, initiation of the malignant process predominantly involves chromosomal rearrangements that activate various oncogenes. Oncogenes as target of new drugs Several oncogenes act in key points of cell life. Most of them, in fact, codify for growth factor receptors or are involved in the signal transduction. Therefore, they represent a natural target for the development of new drugs that are able to block selectively the cells carrying a deregulation in the drug target. Colorectal cancer results from a series of pathological changes that transform normal colonic epithelium into invasive carcinoma. Specific genetic events, shown by vertical arrows, accompany this multistep process. Trastuzumab is indicated for patients affected by a metastatic breast cancer and, more recently, also for the adjuvant and neo-adjuvant setting. Novel inhibitors are currently under evaluation to specifically act against the T790M mutation: these drugs are named "irreversible inhibitors. Results from case reports and from human uveal melanoma cell lines demonstrated that imatinib inhibits cell proliferation and invasion rates. Afterward, it can phosphorylate a substrate that can interact with the downstream ef fector molecules. The Food and Drug Administration approved this drug for the administration to patients carrying the V600E specific mutation, whereas the European Medicine Agency approved it for all the patients carrying any mutation at codon 600 (therefore including V600K, V600D, and V600G, overall accounting for more than 10% of melanoma cases). Most of these have been developed, but many of these showed high toxicity for human cells. Aspirin, in fact, is a drug efficiently preventing colorectal adenomas and carcinomas, and the effect is thought to be driven by the inhibition of cyclooxygenase enzymes. This anticancer effect has reported to be restricted to patients with cyclooxygenases overexpression. Interestingly, this effect could not be observed in patients taken the specific cyclooxygenase inhibitor rofecoxib. One of the most promising aspects of these small-molecule inhibitors in treating cancer is that their targets and mechanisms of action are different from those of cytotoxic drugs and radiation. This makes it feasible to combine small-molecule inhibitors with other treatments, creating a synergistic therapy, without likely development of cross-resistance or increased toxicity. Proto-oncogenes may be activated by mutation, chromosomal rearrangement, or gene amplification. Chromosomal rearrangements that include translocations and inversions can activate proto-oncogenes by deregulation of their transcription. Tumor-suppressor genes, which also participate in the regulation of normal cell growth, are usually inactivated by point mutations or truncation of their protein sequence coupled with the loss of the normal allele. The discovery of oncogenes represented a breakthrough for our understanding of the molecular and genetic basis of cancer. Oncogenes have also provided important knowledge concerning the regulation of normal cell proliferation, differentiation, and programmed cell death. The identification of oncogene abnormalities has provided tools for the molecular diagnosis and monitoring of cancer. Most important, oncogenes represent potential targets for new types of cancer therapies. The goal of these new drugs will be to kill cancer cells selectively while sparing normal cells. One promising approach entails using specific oncogene targets to trigger programmed cell death. Thereafter, a plethora of new targeted drugs has entered clinical trials, with evident benefit for the treatment of several neoplastic disease that were, before targeted therapies development, very hard to be treated and cured. The use of high-throughput technologies for the identification of new oncogenes and the rapidly expanding knowledge of the molecular mechanisms of cancer hold great promise for the development of better combined methods of cancer therapy in the near future. Summary and conclusions the initiation and progression of human neoplasia is a multistep process involving the accumulation of genetic changes in somatic cells. These genetic changes then consist of the activation of cooperating oncogenes and the inactivation of tumor-suppressor genes, both of which appear necessary for a complete neoplastic phenotype. Proto-oncogenes are a diverse group of genes involved in the regulation of cell growth. Proteins encoded by genes involved in chromosomal alterations in lymphoma and leukemia: clinical value of their detection by immunocytochemistry. Mouse mammary tumor virus sequences responsible for activating cellular oncogenes. Gene amplification as double minutes or homogeneously staining regions in solid tumors: origin and structure. Management and future directions in non-small cell lung cancer with known activating mutations. Chromosome translocations in sarcomas and the emergence of oncogenic transcription factors. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Mutations and other alterations in growth promoting genes (oncogenes) and tumor suppressor genes can accumulate during the lifetime of a normal cell resulting in cancer.
Buy discount unisom on line
Over the past four decades sleep aid gift ideas unisom 25 mg with amex, investigations of the molecular mechanisms underlying cancer cell differentiation arrest or blockage have allowed the identification of an array of drug targets. On the other hand, many physiological or pharmacological agents have been tested using in vitro and in vivo systems as the inducers of differentiation and maturation of cancer cells. The translational research in this field has gradually turned the differentiation therapy from a concept to clinical practices. This article discusses the basic theories of differentiation therapy and the clinical achievements of this therapeutic approach. They are often assembled into multiprotein complex with cofactors (activators or repressors) and play decisive roles in regulating the gene expression profiles of stem/progenitor cells and determining their ability to differentiate into mature cell lineages. Notably, many of these receptors are subject to dysregulated expression or structural abnormalities in various cancers. To understand the biology of differentiation arrest or blockage in cancer, it is necessary to elucidate how the related functions are regulated in normal cells and how they become disrupted in cancer cells. It is well established that pluripotent embryonic stem cells are capable of self-renewal and differentiating into various cells with specific functions in an organism. These cells can then develop into distinct cell types, tissues, and organs, which are under accurate regulation in a finely organized manner. Notably, many pathways and networks that regulate cell differentiation during normal development are affected by genetic and epigenetic abnormalities during tumorigenesis. As a result, cancer cell population generally bears an aberrant expression of regulators for embryonic morphogenesis and maintains a subset Holland-Frei Cancer Medicine, Ninth Edition. In addition to self-renewal, normal stem cells are capable of differentiating into specific mature cells, while differentiation blockade may lead to the development of cancer. Cancer stem cells may originate from normal stem cells acquiring genetic and/or epigenetic abnormalities, or from normal progenitor or differentiated cells undergoing de-differentiation and reprogramming also due to genetic and/or epigenetic abnormalities. Differentiation therapy exerts therapeutic efficacy by inducing differentiation of cancer stem cells and undifferentiated cancer cells. However, most current anticancer research activities are concentrated on cell-surface receptors because they offer a relatively straightforward way for drugs to affect cellular behavior, whereas agents acting at the level of transcription need to penetrate the cell membrane and enter into the nucleus. Efforts should be supported to design drugs affecting cancer-associated transcriptional patterns for re-establishing differentiation. Epigenetic modifiers Epigenetic regulation constitutes crucial mechanisms governing the variability in gene expression, which is heritable through mitosis and potentially also meiosis, without changes of genomic sequence. This phenotypic diversity is due to distinct gene expression patterns among different cell types. It is well established that chromatin states are primary determinants for the turn-on or turn-off of genes. Notably, most of these epigenetic modifier genes have been found to be mutated in human cancers including solid tumors and hematological malignancies. Because the epigenetic modifications are usually reversible, therapies targeting epigenetic modifiers hold the promise of being clinically effective. Thus far, about 80 agents of differentiation induction have been identified and biosynthesized, and most of them are under investigations in vitro or on animal models, among which only a fraction have been used in clinical practice. Retinoids have been reported to induce the differentiation of many solid tumor cell lines in vitro and suppress carcinogenesis in tumorigenic animal models, including human teratoma, melanoma, neuroblastoma, osteosarcoma, and rhabdomyosarcoma. These data indicate retinoids as promising differentiation therapy agents for the treatment of thyroid cancer. In addition to induction of differentiation, retinoids exert antitumor effects through other modes of action: repressing cell proliferation, promoting cell apoptosis, and inhibiting angiogenesis and metastasis. Animal model studies have shown that 1,25D strongly promotes cell differentiation and thereby significantly prolongs the survival of mice transplanted with leukemic cells. The clinical utility of 1,25D has also been limited by the severe toxicity of its therapeutic doses, primarily due to fatal drug-induced hypercalcemia. Vitamin D compounds Vitamin D is known to have a variety of actions at cell proliferation and differentiation. Recurrent somatic alterations in epigenetic modifiers are also observed in solid tumors and hematological malignancies. Hence, there has been substantial interest in preclinical and clinical studies for epigenetic regulating agents to be considered as differentiation inducers. Differentiation therapy for cancers, although still in its relative infancy, has given hope to millions of cancer patients. The results have been quickly confirmed by a large amount of clinical practices around the world. Oral arsenic formulations are not currently available in the United States or Europe. The common adverse effects such as minor bone marrow myelosuppression, hepatotoxicity, gastrointestinal reactions, and neurotoxicity can be controlled and are generally reversible without need of the discontinuation of the drug. This scientific history may hence serve as a template for subsequent development of similar treatments in other leukemias and solid tumors. One major challenge is the complexity of histopathological subtypes and clinical stages of cancers, resulting in the absence of developmental models of cancer progression. The re-establishment of the genotype that characterizes the original tissue types and the morphological transformation of tumor cells to the normal cells may help to determine more successful differentiation induction. In addition, there have not yet been definite markers for evaluating the effects of differentiation inducers. Assessment of the precise therapeutic role of agents is often hampered by the difficulties in distinguishing in vivo cytotoxicity from differentiation. The classic evaluation of therapeutic responses mainly focuses on the shrinkage of the tumor mass, but this is not suitable for the response evaluation of differentiation therapy that just restores the differentiation program of tumor cells. Consequently, identifying accurate novel biomarker sets of response to differentiation therapy becomes urgent for clinical application. Nevertheless, solid tumors are still viewed as much more heterogeneous aberrant tissues than most leukemia, and much more complex molecular mechanisms are involved in their pathogenesis. Cancer cells may be blocked at a particular stage of differentiation along with the involved cellular lineage, or they may differentiate into an inappropriate cell type. Hence, cancer differentiation therapy represents the approach aimed at the re-activation of endogenous differentiation programs or subverting differentiation/maturation blockage within cancer 158 Tumor biology cells, often accompanied by the loss of malignant phenotype and the restoration, at least in part, of the normal phenotype. The concept of differentiation therapy was pioneered by the work of Pierce and Verneyin 1961 when they observed differentiation of teratocarcinoma cells. To trigger malignant cells to overcome their differentiation block and to enter the apoptotic pathways has become an elegant alternative to the therapies simply killing malignant cells. However, differentiation therapy had historically been hampered by many factors, especially due to the insufficient understanding of the pathways of normal cell differentiation and the much higher complexity of the process of cancer cell reversion to "normal" cells/tissues induced by differentiation therapy than the cytotoxic approaches. This article focuses on the basic theories of differentiation therapy, and the clinical achievements of this novel therapeutic approach. Hacking cell differentiation: transcriptional rerouting in reprogramming, lineage infidelity and metaplasia. Understanding and targeting cancer stem cells: therapeutic implications and challenges. The 12th international conference on differentiation therapy: targeting the aberrant growth, differentiation and cell death programs of cancer cells. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Key clinical observations after 5-azacytidine and decitabine treatment of myelodysplastic syndromes suggest practical solutions for better outcomes. Recent progress toward epigenetic therapies: the example of mixed lineage leukemia. Retinoic acid treatment of acute promyelocytic leukemia: in vitro and in vivo observations. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol.
Discount unisom online master card
One is to assume that r1 and r2 are unrelated and to base any inferences about r2 on the results of the second trial alone insomnia hormones purchase unisom 25 mg overnight delivery. In a Bayesian hierarchical model, both possibilities are allowed, but neither is assumed. In other words, r1 and r2 are regarded as having come from a population of r-values. The population may have little variability (homogeneity) or substantial variability (heterogeneity). The observed response rates give information concerning the extent of heterogeneity, with disparate rates suggesting greater heterogeneity. When the observed rates are similar, the precision of estimates of r1 and r2 will be greater than when the observed rates are disparate. In the former case, there will be greater "borrowing of strength" across the trials. If it happens that the results of the trials are very different, then there will be little borrowing and the information from any one trial will not apply much beyond that trial. More generally, there may be any number of related studies or databases that provide supportive information regarding a particular therapeutic effect. The next example is generic but it is more complicated than the previous example because it includes nine studies. The dot plot on the r-axis shows the observed response proportions given in Table 2. The pooled-analysis curve shows the distribution of response rate r assuming no study effect. The hierarchical analysis curve shows the Bayesian estimate of the distribution of response rates allowing for heterogeneity across the various studies. Each of the nine sample response proportions S/n is an estimate of the corresponding r. However, the precision associated with this estimate is too great (equivalently, its standard error is too small). This is also the predictive probability of the response for a future patient in that study. This shrinkage is greater for smaller studies, and for studies with observed proportions further from the overall mean. Hierarchical borrowing is defensible because it does not make the assumption that all studies had the same true response rate, and because the extent of borrowing is determined by the data. The Bayesian estimates are intermediate between simple pooling (complete shrinkage) and each trial standing alone. The amount of shrinkage- including the two extremes mentioned previously-depends on the prior distribution of the population of trials. This aspect of the prior distribution should be set in advance, or varied to allow for assessing the sensitivity of the overall conclusion. The motivation for such modeling is to use the available information appropriately in improving precision or in decreasing the required sample size. Simply pooling the data from the other eight studies would greatly increase the precision of its estimated response rate. However, in view of the possibility of heterogeneity in the studies, such pooling would 262 Quantitative oncology Table 2 Numbers of responses S, sample size n, observed response proportions (including its standard error), and adjusted estimates of response rates by study. Study number Responses S Sample size n Observed response proportions (standard error) 0. The Bayes estimates assume a hierarchical model and show shrinkage toward the overall mean. In other words, the savings in terms of sample size cannot be predicted with certainty. The interim results can be used to determine the precision associated with the estimates of the various response rates. If the interim results will not be available when the decision to stop the trial must be made, then the uncertainties regarding heterogeneity across subtypes can be assessed at the trial design stage and the sample size chosen accordingly, recognizing that the eventual precisions cannot be predicted perfectly. In this section, we focus on a narrow aspect of survival analysis that enables greater understanding of cancer and its treatment. We provide no p-values for the comparisons (high vs moderate; high vs low) because the statistical significance is not relevant to our purpose. Hazards are the proportions of events within a particular time period as a fraction of those patients who are at risk at the beginning of the period. For example, suppose that the event is a recurrence and there are 100 patients in a group that are at risk in the first year. If 10 of these patients experience a recurrence of the disease in the first year, then the first-year hazard is 10%. If another 18 experience recurrences in the second year, then the second-year hazard is 18/90 = 20%. Although smaller than the reduction with unabashed pooling, this reduction implies that >50% savings in sample size is necessary to carry out a clinical trial (in the setting of study number 1 in Table 2) with the same precision: (0. For example, to achieve the same standard error in a stand-alone study would require 25 as opposed to 16 patients in study number 1 of Table 2. Patient covariates can be incorporated into a hierarchical analysis, thus adjusting for known differences in the studies but still accounting for unknown effects. In this example and in more complicated hierarchical settings as well,21 modeling allows for borrowing from other studies and databases. If the results are consistent across studies, then the amount of borrowing will be greater. If the results are sufficiently different (after accounting for covariates), then this suggests heterogeneity among the studies and there is little borrowing. Hierarchical modeling in trial design There are many settings in which trials can be set up to borrow strength from related, but not necessarily identical, experimental units. Consider designing a trial for a therapy for a disease that has several subtypes, such as several different histologies that exist for one type of tumor. Recognizing the possibility of borrowing across subtypes means greater precision for estimating each individual response rate and therefore that the sample size within each subtype can be smaller. Each incremental hazard prediction depends on the data for the corresponding year. Principles of statistical design: decision analysis and factorial experiments Decision analysis Clinical practice and clinical research involve making decisions. A list of the possible results, probabilities, and consequences of each decision, pros and cons expressed as utilities, allows for choosing one decision over another. Predictive probabilities play a central role in the decision-making process, which makes the Bayesian approach ideal. Choosing the trial design and selecting the optimal sample size are decisions that benefit from the use of decision analysis, which is described in greater detail in other references. The patients in both treatment arms may receive additional therapy based on the current standard of care for the type and stage of their cancer, and on their prior treatment. They may have surgery and receive concomitant radiation and other chemotherapy, which may include several drugs. If the experimental drug is shown to be sufficiently effective, then it will be incorporated into the standard therapy. One is that it is not possible to assess the individual contributions of the various components of polychemotherapy or combination therapy that are developed in this way. Another is that the approach provides a mechanism for adding drugs to standard regimens but not for subtracting them. Adding an experimental drug to a standard regimen, however, may make other components of the regimen unnecessary. Studying the possible removal of a component of standard chemotherapy is difficult, and for ethical reasons this is so, even if the component has never been proven to contribute to the overall effectiveness of the combination. However, better approaches for developing drugs would alleviate the problem and could lead to more rapid development of better therapies. A fundamental principle of optimal experimental design in statistics is to change the various contributing factors in such a way as to learn efficiently about their impact on outcome. This impact may involve interactions between the factors and it is important to learn about such interactions. What is required is to model relationships and exploit the available data to inform the model. An alternative to varying one factor at a time in separate studies is using factorial designs.
Cheap unisom express
While there are over 113 gene variants sleep aid dollar tree purchase 25 mg unisom free shipping, only several variants have been described to influence irinotecan pharmacodynamics (*6, *27, *28, *36, *37, *60, *93). This article focuses on *28 and *6 variants, which are clinically relevant with supporting studies. Numerous meta-analyses have evaluated the association of *28/*28 genotype to the risk of toxicity as a function of irinotecan dose. Doxorubicin is characterized by substantial interindividual variations in pharmacokinetic parameters182 and cumulative dose is the most significant risk factor for doxorubicin-induced cardiotoxicity. This is likely due to different patient populations, other covariants, and individual substrate drug. One small study in Asian patients noted impaired doxorubicin pharmacokinetics, resulting in significantly increased exposure levels and reduced clearance; however, the study involved only a small number of patients. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. The important role of albumin in determining the relative human blood stabilities of the camptothecin anticancer drugs. Phase I and pharmacologic study of topotecan in patients with impaired renal function. Daunorubicin, epirubicin, etoposide Limited data are available on pharmacogenomics of daunorubicin, epirubicin, or etoposide. It is important to identify the molecular basis of sensitivity and resistance so that in the future, strategies can be developed for making optimal therapeutic choices. Phase I and pharmacologic studies of topotecan in patients with impaired hepatic function. A comparison of clinical pharmacodynamics of different administration schedules of oral topotecan (hycamtin). Topotecan and cytarabine is an active combination regimen in myelodysplastic syndromes and chronic myelomonocytic leukemia. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Evaluation of topotecan in resistant and relapsing multiple myeloma: a Southwest Oncology Group Study. Billions of years of evolutionary pressure have resulted in the natural selection of plants, fungi, and microorganisms capable of producing potent and specific toxins. After several plant-derived compounds and other natural products, many of which targeted the mitotic processes, demonstrated prominent anticancer activity in the 1950s and 1960s, the microtubule was recognized as a subcellular target of major strategic importance. The first widely used class of antimicrotubule agents, the plant-derived vinca alkaloids, had been the mainstay of both palliative and curative regimens for treating malignancies for several decades. More recently, several plant- and marine-derived compounds as well as synthetic agents with yet even more distinctive disruptive actions on microtubules and other mitotic constituents have been identified. These include the analogs of the epothilones and halichondrin B, which were isolated from soil-dwelling myxobacterium and marine sponges, respectively. They also include potent antimicrotubule agents, such as analogs of maytansine and dolastatin, which are components of antibody-drug conjugates. This article focuses on the microtubule as a target for therapeutic development and antimicrotubule agents that comprise our therapeutic armamentarium. The addition of the plant-derived taxanes, which possess a unique mechanism of action and anticancer spectra, to our therapeutic arsenal several decades later resulted in renewed interest in the microtubule and mitotic processes as targets for which to develop cancer therapeutics, as well as in the identification of other natural products to treat cancers. More recently, several plant- and marine-derived compounds as well as synthetic agents with yet even more distinctive disruptive actions on microtubules and other mitotic constituents. This article focuses on the microtubule as a target for therapeutic development and antimicrotubule agents that comprise our therapeutic armamentarium, particularly the vinca alkaloids and taxanes, as well as several classes of promising antimicrotubule agents undergoing clinical evaluation. Microtubules as strategic targets against cancer Microtubules are highly regulated and integral components of the cellular cytoskeleton that can be disrupted by various natural products. Although the most important functions of microtubules in proliferative cells are through their actions as components of the cytoskeleton and mitotic spindle apparatus, which pulls apart chromosomes and is vital to cell division, they are involved in many other critical functions throughout the cell cycle, including intracellular transport of vesicles and organelles, trafficking of proteins including many oncoproteins, locomotion, adhesion, and anchorage of subcellular organelles and receptors. The specific expression of transcription factors in concert with drug-mediated depolymerization of microtubules has been well described and such has provided information on the differential expression of specific genes. In essence, billions of years of evolutionary pressure have resulted in the natural selection of plants, fungi, and microorganisms that are capable of producing highly potent and specific toxins. After several plant-derived compounds and other natural products, many of which were noted to suspend cell division in mitosis by affecting the mitotic spindle, demonstrated prominent anticancer activity in patients with advanced malignancies in the 1950s and 1960s, the microtubule was recognized as a subcellular target of major strategic importance. The first widely used class of antimicrotubule agents, the plant-derived vinca alkaloids, had been the mainstay of both palliative and curative regimens for treating malignancies for Holland-Frei Cancer Medicine, Ninth Edition. Typically, microtubules are formed by the parallel association of 13 protofilaments, although microtubules composed of fewer or more protofilaments have been observed in vitro. Microtubules have distinct polarity, which is conferred by the unique alignment of the protofilaments. Therefore, one end of a protofilament will have the -tubulin subunits exposed, while the other end will have the -tubulin subunits exposed. The protofilaments align parallel to one another with the same polarity, so, in a microtubule, there is one end, the plus end, with only -tubulin subunits exposed, while the other end, the minus end, has only -tubulin subunits exposed. The unique functions of microtubules are related to their polymerization dynamics, involving a dynamic equilibrium between an intracellular pool of /-tubulin dimers and microtubule polymers, and simultaneous release of the /-tubulin dimers into the soluble tubulin pool. The dynamic equilibrium between free /-tubulin dimers and the microtubule occurs simultaneously at both ends of the microtubule. Although tubulin polymerization and dissociation, and consequently microtubule elongation and shortening, occur simultaneously at each end of the microtubule, the net changes in length at the more kinetically dynamic plus end are much larger over time than those at the minus end. If the polymerization reaction is followed in vitro, an initial lag phase is noted, after which microtubules form rapidly until a plateau phase is reached. The dimers associate linearly to form protofilaments that then in turn associate laterally to form the hollow cylindrical wall of the microtubule. Protofilaments can twist slowly around the microtubule axis, although these shown here are in parallel as in microtubules containing 13 protofilaments. Monomers of each type thus are in contact along a shallow spiral path around the microtubule. Treadmilling and dynamic instability Two principal processes govern microtubule dynamics in live cells. The second dynamic process, termed dynamic instability, occurs when the plus ends of microtubules switch spontaneously between states of slow sustained growth and rapid shortening. This complex acts as a template for /-tubulin dimers to begin polymerization; it caps the negative end, while microtubule growth occurs at the free positive end. The rate of dynamic instability is accelerated during mitosis, resulting in the formation and attachment of the mitotic spindles to the chromosomes. In most cells, mitosis progresses rapidly, and the highly dynamic microtubules that comprise the mitotic spindle render them sensitive to antimicrotubule agents that disrupt polymerization dynamics. Dynamic instability and treadmilling enable the microtubules of the mitotic spindle to make vast growing and shortening excursions, often termed search and capture, essentially probing the cytoplasm, until their positive ends become attached to a chromosome at its kinetochore. If even a single chromosome is unable to achieve a bipolar attachment to the spindle, perhaps because of drug-induced suppression of microtubule dynamics, the cell will not traverse beyond a prometaphase/metaphase-like state and instead will undergo apoptosis due to a complex series of processes involving the spindle assembly checkpoint. In anaphase, microtubules attached to the chromosomes undergo shortening, while another subpopulation of microtubules, called interpolar microtubules, lengthen, resulting in polar movement of the chromosomes. Suppression of spindle-microtubule treadmilling and dynamic instability by antimicrotubule agents reduce spindle tension and impede progression from metaphase to anaphase, thereby triggering cell death. There are at least six isotypes of -tubulin and -tubulin each in human cells, which are distinguished by different C-terminal amino acid sequences and encoded by a large multigene family that has been highly conserved throughout evolution. Posttranslational modifications generally occur on the C-terminal region of -tubulin. This region, which is rich in negatively charged glutamate, forms relatively unstructured tails that project out from the microtubule and contact motor proteins. Therefore, posttranslational modifications appear to regulate the interactions of motor proteins with microtubules. Vinca alkaloids: Introduction and indications the vinca alkaloids are naturally occurring or semisynthetic nitrogenous bases extracted from the pink periwinkle plant Catharanthus roseus G. The early medicinal uses of this plant led to screening these compounds for their hypoglycemic activity, which was ultimately of minor importance compared to their cytotoxic effects.
Diseases
- Symphalangism brachydactyly
- Subpulmonary stenosis
- Tyrosinemia
- Hemochromatosis type 3
- Pemphigus and fogo selvagem
- Acute idiopathic polyneuritis
Generic unisom 25 mg visa
For example insomnia jason derulo buy 25mg unisom visa, the Survivin protein plays a fundamental role in chromosome segregation and cytokinesis. Many members of the Bcl-2-family have a hydrophobic stretch of amino acids near their carboxyl-terminus that anchors them in the outer mitochondrial membrane. In contrast, other Bcl-2-family members such as Bid, Bim, and Bad lack these membrane-anchoring domains, but dynamically target to mitochondria in response to specific stimuli. Still others have the membrane-anchoring domain but keep it latched against the body of the protein, until stimulated to expose it. Based on their predicted (or experimentally determined) three-dimensional structures, Bcl-2 family proteins can be broadly divided into two groups. One subset of these proteins is probably similar in structure to the pore-forming domains of bacterial toxins, such as the colicins and diphtheria toxin. Bcl-2 has also been proposed to regulate mitochondrial redox metabolism, though mechanistic details remain largely unclear. Anti-apoptotic protein Bcl-2 binds the autophagy protein Beclin, preventing it from forming complexes with downstream autophagy proteins. These recent findings concerning links between autophagy, tumorigenesis, and anti-apoptotic Bcl-2-family proteins indicate a master-switch role for Bcl-2-family proteins in controlling cell life and death decisions. Genomic lesions involving members of the Bcl-2 family are well documented in human cancers. Conversely, mutations inactivating pro-apoptotic members of the Bcl-2 family also occur in some human cancers (Bax). In addition, a wide and diverse array of epigenetic mechanisms contribute to dysregulation of Bcl-2 family gene expression in cancers and leukemias, some of which are outlined below. Signal transduction pathway alterations in cancers-impact on cell death machinery Various receptor-mediated signal transduction pathways converge on the core components of the cell death machinery outlined above, including tyrosine kinase growth factor receptors and their downstream signaling pathways that become deregulated in many cancers. The murine gene encoding Akt was first discovered by virtue of its similarity to the v-akt oncogene found in some murine leukemia viruses, where it becomes activated in thymomas caused by retrovirus insertions near the c-akt gene. Illustrative examples of the connections to apoptosis-regulating proteins and genes are shown, without attempting to be comprehensive. For example, phosphorylation by Erk of pro-apoptotic protein Bim on serine 69 promotes Bim protein degradation via proteasomal-dependent mechanisms. However, acetylation of non-histone proteins is increasingly recognized as a mechanism for impacting biological processes through diverse mechanisms. In terms of cell death regulations, examples of dysregulated protein acetylation impacting the core components of the cell death machinery are only just emerging. Among the cancer-relevant connections are steroid hormone receptors including estrogen, androgen, and vitamin D receptors, which regulate the expression of various cell death and cell survival genes. Precedence also exists for nontranscriptional mechanisms of regulating cell death proteins, where transcription factors leave the nucleus and physically interact with Bcl-2 family members on Cancer and cell death 175 the surface of mitochondria. Therefore, cancer-associated aberrations in the regulation of transcription factors and chromatin-modifying enzymes are connected ultimately to perturbations in the core cell death machinery. The cellular injuries induced by classical chemotherapeutic anticancer drugs, and x-irradiation, trigger molecular events with connections to the core cell death machinery. Cytoprotective proteins that blunt cell death pathways thus play important roles in chemoresistance. The p53 protein is a tetrameric transcription factor, whose levels are controlled by the E3 ligase Mdm2. Small-molecule drugs that block Mdm2 protein interaction with p53 have shown promising preclinical activity against hematological malignancies144 and are synergistic with Bcl-2 inhibitors. For example, the pro-apoptotic Bcl-2 family member Bmf-1 is sequestered on microtubules. When microtubules are disrupted by anticancer drugs, Bmf is released from microtubule-binding proteins (dynein light chains), allowing it to Cancer drug discovery by targeting the cell death machinery Multiple efforts have been made to devise therapeutics that neutralize anti-apoptotic or activate pro-apoptotic components of the core cell death machinery. The relative attractiveness of these alternative strategies depends on the nuances of the target and its biology. Simultaneous overexpression of two or more anti-apoptotic members of the Bcl-2 family in tumors has also been documented in some cases, raising the issue of redundancy. Thus, restoring chemosensitivity by nullifying Bcl-2 and related proteins has emerged as an attractive strategy for improving cancer therapy. Chemicals that bind regulatory sites on anti-apoptotic Bcl-2family proteins and that directly neutralize the cytoprotective actions of these proteins have been identified and brought into clinical development. Some have progressed into advanced human clinical trials, and at least one molecule (venetoclax) has produced sufficiently compelling data that the U. Other approaches to tackling Bcl-2 family proteins for cancer therapy have been proposed, though most of those concepts are less validated. For example, direct agonists of Bax and Bak that mimic the actions of Bid, Bim, and Puma could be envisioned,166 though this has not been reduced to practice to date. Alternatively, indirect mechanisms for upregulating pro-apoptotic Bcl-2 family members are actively being explored. Mdm2 antagonists have progressed to advanced clinical trials, following displays of promising activity for some types of malignancy such as acute leukemia. It remains to be seen whether the Mdm2 inhibitor strategy is applicable only to tumors that retain wild-type p53 versus whether it may also benefit some patients whose malignancies harbor mutants of p53 that still retain the ability to exit the nucleus and directly modulate Bcl-2 family protein activity at the surface of the mitochondria (see above). In an analogous fashion, small-molecule inhibitors of Akt that are currently in advanced clinical development also would be expected to help restore the activity of pro-apoptotic Bcl-2 family proteins that are phosphorylated by this protein kinase. More recently, highly engineered antibodies that utilize FcR-independent mechanisms to achieve death receptor crosslinking have been advanced into clinical studies, awaiting results at the time of this writing. The possible advantage of mitochondria-independent apoptosis is that roadblocks to apoptosis created by survival proteins such as Bcl-2 are bypassed, an attractive feature for chemorefractory cancers that often have defects in the mitochondrial pathway. Several of the synthetic compounds were taken into clinical development, and some of them remain actively under investigation at the time of this writing. More extensive comparisons of the therapeutic indices of selective versus broad-spectrum inhibitors are needed to reveal the best path forward. Classification of cell death: recommendations of the nomenclature committee on cell death. The permeability transition pore complex: a target for apoptosis regulation by caspases and Bcl-2-related proteins. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. In several cases, the available information has already sparked efforts to translate the base of information into therapeutic strategies, with new agents for cancer treatment nearing or already reality. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Shay, PhD Overview Finding specific targeted agents for cancer therapy remains a challenge. One of the hallmarks of cancer is the limitless proliferation (immortalization) of cancer cells, which correlates with the activation of the ribonucleoprotein enzyme complex called telomerase. Although there have been several approaches developed for telomerase-targeted therapies, there is still no agent that has been approved. This article focuses on the pros and cons of the different approaches to target telomerase and summarizes preclinical and clinical results. We will also review novel telomerase-mediated telomere uncapping approaches that target telomerase-expressing cancer cells, but not normal cells. It is believed that only a few short telomeres with uncapped ends are sufficient to trigger a p53-dependent G1/S cell cycle arrest, which is known as replicative senescence. When cells enter senescence, the fate of normal cells is to remain in a quiescent phase. Senescence and crisis are two fundamental mechanisms that protect cells against the early development of cancer. There have been several studies on changes in telomerase expression/activation in normal and cancer cells. This process is known alternative splicing16 and may be important in the regulation of telomerase.
Purchase unisom 25 mg overnight delivery
Autologous transplants require no histocompatibility matching but often come from patients who have received extensive prior chemotherapy insomnia 2 am buy cheap unisom 25mg line. This treatment often compromises marrow stem cell function making adequate stem cell collection more difficult. The recipient receives a myeloablative preparative regimen designed to eradicate malignant and normal cells. Tumor contamination of collected cells represents a possible source of relapse and patients with significant tumor infiltration of marrow may be less desirable autotransplant candidates. Patients undergo collection of bone marrow or peripheral blood progenitor cells that are then cryopreserved in a viable state. Patients subsequently receive high-dose myelosuppressive therapy to eradicate the malignancy, followed by reinfusion of the stored cells to reconstitute hematopoiesis. Autologous hematopoietic cells can potentially be used as vehicles for gene therapy designed to correct deficiency states. Allogeneic transplantation Allogeneic transplants are collected from related or unrelated donors. Registries of potential unrelated donors have been established to provide hematopoietic transplants for patients lacking a histocompatible relative. Linkage disequilibrium occurs such that some haplotypes occur commonly, but approximately half of the population have rare haplotypes. Ten-antigen matched unrelated donor transplants now have outcomes that approximate those for histocompatible sibling donors. Delayed immune reconstitution and a high frequency of opportunistic infections remain major complications of both these techniques. Like autotransplantation, allogeneic cells are usually administered after an intensive chemotherapy program. In addition to tumor killing, these programs are also designed to produce immunosuppression sufficient to facilitate engraftment of the allo cells. The intensity required is, in part, proportional to the immunocompetence of the recipient. In some circumstances, reduced-intensity chemotherapy is used to decrease regimen-related toxicity or to produce a mixed chimeric state. A subsequent graft-versus-hematopoietic effect may then occur, which eradicates residual normal and malignant cells of host origin, resulting in complete chimerism (presence of only donor derived hematopoietic and immune cells). Immune reconstitution is more rapid than after an allogeneic transplant and there is a lower risk of opportunistic infections. Treatment-related mortality is lower than 5% in most studies, and elderly patients can tolerate treatment relatively well. In most malignancies, relapse rates are higher after autologous transplants than after allogeneic transplantation, although this is often offset by a lower rate of treatment-related mortality. Patients with extensive prior therapy are at high risk for developing myelodysplasia and secondary acute leukemia after autologous hematopoietic transplantation. Immune reconstitution is slower after allogeneic transplantation and opportunistic infections are more frequent. Treatment-related mortality is significantly higher than with autologous transplantation. The outcome of transplantation relates to the selection of patients and timing of transplant during the natural history of the malignancy. The best results occur when the transplant is performed early in the disease course, when the malignancy is still sensitive to chemoradiotherapy treatment, and when the tumor burden is low. Conversely, transplants done as a last resort are associated with high rates of both relapse and treatment-related toxicity. Responsiveness to conventional-dose chemotherapy is a major predictive factor for the outcome of hematopoietic transplant. The best results have been achieved in patients with chemosensitive relapse or when transplant is performed in high-risk patients as consolidation of response, particularly in patients with minimal disease at the time of transplant. However, patients with bulky disease, refractory relapse, or multiple relapses of their malignancy have a poor prognosis. Alkylating agents are the most commonly used drugs in transplant regimens because they usually kill tumor cells in proportion to increasing dose. In recent years, monoclonal antibodies have proven to be useful when added to standard treatments for leukemia and lymphoma. Hematopoietic cell transplantation 835 these regimens can be tolerated by older patients and those with comorbidities who are not eligible to receive an ablative preparative regimen. Engraftment has been achieved in most patients, and this approach is effective against a range of hematologic malignancies. A maculopapular rash is usually the first presentation and is typically pruritic and confluent. Results are improving for unrelated donor transplantation with the use of more precise molecular histocompatibility typing to identify donors. The current standard of care combines either cyclosporine or tacrolimus with a short course of methotrexate. Approximately half of patients have a sustained response,91 and the steroid dose can be gradually tapered off. For mismatched grafts, recent studies have demonstrated that the use of post-transplant cyclophosphamide can decrease the Complications of hematopoietic transplantation Intensive chemoradiotherapy and hematopoietic transplantations may be associated with a number of serious complications as listed in Table 2. Graft rejection and graft failure Graft failure is defined as the failure to establish hematopoietic engraftment (primary graft failure) or loss of an established graft (secondary graft failure). Graft failure after autologous transplant occurs rarely and is most often related to infusion of an inadequate number of viable stem cells. Graft failure after allogeneic transplantation is also caused by immunologic graft rejection. Alternate day dosage may be preferable to minimize the complications resulting from chronic steroid therapy. Cyclosporine or tacrolimus may be used in combination with corticosteroids in high-risk patients. The actual risk for toxicity varies among regimens and their relative dose intensity. Specific determinants include the toxicity profiles of the involved agents and their interactions that are affected by coexisting organ dysfunction, the effects of the diseases and prior therapy, and infections. Localized skin involvement and/or hepatic dysfunction, plus any of the following: (a) liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis (b) eye involvement (Schirmer test <5 mm wetting) (c) involvement of mucosalivary glands or oral mucosa (d) involvement of any other target organ Extents determined by rule of nines or burn chart. Immunodeficiency and infections Recipients of hematopoietic transplants have a severe immunodeficiency involving both T and B cells. Recipients of autologous and syngeneic transplants also have a period of immunodeficiency but recovery is more rapid. Recipients of hematopoietic transplants may be susceptible to unusual opportunistic infections and to acute overwhelming infections. Prophylactic strategies against an array of potential infections and rapid recognition and treatment of infections are an essential part of successful management of transplant recipients. Revaccinations should be performed upon immune recovery and are typically carried out 6 months posttransplant. Treatment includes withdrawal of immunosuppression and administration of rituximab. Late toxicity of high-dose therapy can produce cataracts, pulmonary fibrosis, dental abnormalities, hypothyroidism, hypogonadism, growth retardation, osteoporosis, and avascular necrosis of the hip or other bones. There is an increased risk of solid and hematologic secondary tumors after hematopoietic transplantation. Most patients achieve complete remission after high dose therapy and hematopoietic transplantation. For patients with a favorable prognosis such as those with t8;21, t15;17, or inv 16 abnormalities, transplant is usually deferred until relapse and reinduction of remission.
Purchase unisom 25 mg mastercard
Compared to neoadjuvant chemotherapy trials insomnia doctors generic unisom 25 mg line, the neoadjuvant studies exploring the effect of endocrine therapy in the treatment of breast cancer are much smaller. Several trials have compared neoadjuvant tamoxifen to a third-generation aromatase inhibitor among postmenopausal women with hormone receptor-positive breast cancer. However among the 124 women considered to require a mastectomy at baseline, 46% treated with anastrozole were considered to be candidates for breast-conserving surgery by their surgeon, compared with 22% receiving tamoxifen (p = 0. Considering the data described and the better side effect profile compared to tamoxifen current guidelines recommend considering the use of aromatase inhibitors among postmenopausal women who are at moderate to high risk of developing breast cancer. Cardiovascular disease Postmenopausal women with breast cancer may be at a higher risk of cardiovascular events due to their age, menopausal status, associated comorbid conditions, and exposure to chemotherapeutic agents used in the treatment of breast cancer. As described earlier, tamoxifen, through its estrogen agonist function, has been shown to have a lipid-lowering effect that has translated into modest reductions in cardiovascular events. Although longer follow-up will be required to assess the cardiovascular effects of the clinically used third-generation aromatase inhibitors, women with breast cancer are in general at higher risk of developing a cardiovascular event due to a multitude of factors and as such should monitored and managed appropriately. Other adverse events Another important side effect of aromatase inhibitors is the development of arthralgia which can significantly impact quality of life. The investigators noted that compliance with anastrozole gradually decreased over time with a significant association observed between arthralgia mean scores and noncompliance. Other side effects commonly associated with aromatase inhibitors include vaginal dryness and dyspareunia. Although treatment with aromatase inhibitors increases the risk of vasomotor symptoms and vaginal bleeding/discharge, large trials have shown that the incidence of these events are lower compared to that experienced on tamoxifen treatment. Resistance to endocrine therapy We have certainly come a long way in the management of women with hormone receptor-positive breast cancer in both the adjuvant and metastatic setting. However although endocrine therapy is effective in the metastatic setting, only 30% of patients with hormone receptor-positive breast cancer have an objective response with approximately only 50% gaining clinical benefit from endocrine therapy. In the adjuvant setting clinical trials have demonstrated that approximately fifty percent of patients with hormone receptor-positive breast cancer benefit from endocrine therapy, while the rest develop either a primary or acquired resistance. With an increased understanding of the mechanisms of resistance, selective targeted inhibitors have been introduced which when used in combination with endocrine therapy can overcome resistance. The addition of everolimus to exemestane increased toxicities including stomatitis (grade 3 8% vs 1 %), pneumonitis (grade 3, 3% vs 0%), liver dysfunction, and hyperglycemia. At present the combination of everolimus and exemestane has been recommended for use among patients whose disease has progressed on a prior aromatase inhibitor. Synergistic growth inhibitor effect was seen with the combination of tamoxifen and palbociclib in a model with acquired tamoxifen resistance. Grade 3/4 neutropenia (54% vs 1%) and fatigue (4% vs 1%) were reported with higher frequency in the palbociclib/letrozole group compared to the single-agent letrozole group. The investigators reported a significant improvement in the progression-free survival (9. Among women who receive chemotherapy those younger than age 35 years, the long-term incidence of amenorrhea is at approximately 10%, while it is approximately 50% among women between the ages of 35 and 40 years and can be up to 85% among those above age 40 years. Tamoxifen has been ubiquitous as the frontline therapy for the treatment of all stages of breast cancer and remains the central choice for the treatment of premenopausal women. Emerging data suggests that the use of aromatase inhibitors with ovarian function suppression among premenopausal women with early-stage breast cancer who are at high risk of recurrence may be associated with improved prognostic outcome. Among postmenopausal women the introduction of the noncross-resistant aromatase inhibitors has changed recommendations being now at the forefront of treatment of both early and 732 Chemotherapy advanced staged breast cancers. However several questions regarding the use of aromatase inhibitors still remain including duration of use in the adjuvant setting and sequence of use with tamoxifen. Moreover among the three third-generation aromatase inhibitors, there are no head to head comparisons to support the superiority of one aromatase inhibitor over another. Furthermore the use of aromatase inhibitors are not without side effects with various methods available. We have also come a long way in understanding mechanisms underlying the development of endocrine resistance with agents such as everolimus now approved for use in combination aromatase inhibitors. Lastly, as more antihormonal therapies become available and our understanding of the molecular pathways underpinning resistance increases, it is essential that the optimal sequence of endocrine agents be established in the treatment of breast cancer. This may prolong the time during which endocrine therapies can be used, so postponing the time when cytotoxic chemotherapy becomes a necessary option. An individual patient-based meta-analysis of tamoxifen versus ovarian ablation as first line endocrine therapy for premenopausal women with metastatic breast cancer. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Effect of luteinizing hormone- releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy. Laboratory and clinical investigations have identified a plethora of drug-resistance mechanisms, some that are particular to an individual agent and others that are generalizable across classes of agents. These mechanisms include altered cellular accumulation and detoxification of drugs, mutation of the drug target, change in expression of the drug target, and activation of alternative signaling pathways. The identification of drug-resistance mechanisms has led to strategies to overcome resistance and improved clinical outcomes. Exceptions include emergence of cross-resistance to multiple, apparently structurally and functionally unrelated drugs, to which the patient or cancer cells were never exposed during the initial drug treatment. A more comprehensive discussion of selected mechanisms of resistance to specific classes of drugs will be discussed in subsequent sections. Decreased drug accumulation Decreased intracellular levels of cytotoxic agents is one of the most common mechanisms of drug resistance. As polar, water-soluble drugs cannot penetrate the lipid bilayer of the cell membrane and require specific mechanisms of cell entry, resistance to these drugs is readily mediated by downregulation of drug uptake mechanisms in tumor cells. For example, decreased conversion of nucleobase analogs to their cytotoxic nucleoside and nucleotide derivatives by alterations in specific kinases and phosphoribosyl transferase salvage enzymes can lead to resistance to these anticancer drugs. For example, optimal formation of inhibitory complexes between 5-fluorodeoxyuridine Systemic therapy with cytotoxic drugs or targeted agents is the basis for most of the effective treatments of disseminated cancers. Additionally, adjuvant chemotherapy can offer a significant survival advantage to selected patients, following the treatment of localized disease with surgery or radiotherapy, presumably by eliminating undetected, minimal, or microscopic residual tumor. However, the responses of tumors to chemotherapeutic regimens vary, and failures are frequent owing to the emergence of drug resistance. The phenomenon of clinical drug resistance has prompted studies to clarify mechanisms of drug action and to identify mechanisms of antineoplastic resistance. It is expected that through such information, drug resistance may be circumvented by rational design of new noncross-resistant agents, by novel delivery or combinations of known drugs, and by the development of other treatments that might augment the activity of or reverse resistance to known antineoplastics. While earlier mechanisms of drug resistance were identified experimentally by generation of resistant cell lines, recent advances in genomic technology have allowed the direct determination of resistance mechanisms present in clinically resistant tumors. General mechanisms of resistance to single agents Experimental selection of drug resistance by repeated exposure to single antineoplastic agents will generally result in cross-resistance to some related agents of the same drug class. This phenomenon is explained based on shared drug transport carriers, drug metabolizing pathways, and intracellular cytotoxic targets of these structurally and biochemically similar compounds. Generally, the resistant cells retain sensitivity to drugs of different classes with alternative mechanisms of cytotoxic action. Increased rate of detoxification the manner in which cells metabolize cancer drugs and other xenobiotics is often described as involving three phases of detoxification. Although none of these phases are obligatory steps in the metabolism of every drug, the concept represents a useful framework with which to view cellular detoxification mechanisms. Alterations in any of these three phases can influence the sensitivity or resistance to a particular drug or xenobiotic toxin. Generally, the drug or xenobiotic is rendered into a more electrophilic, reactive intermediate-a process that may enhance toxicity.
25 mg unisom with visa
Side effects and dosing of progestin the most common adverse effect of progestin is weight gain insomnia jobs best purchase unisom, which occurs as a result of increased appetite and fluid retention. Its appetite-stimulating effect has frequently been used to treat cancer-induced cachexia. Other reported side effects include hot flashes, sweating, vaginal bleeding, nausea, dyspnea, thromboembolism, and rare cardiovascular events such as heart failure. Aromatase catalyzes three separate steroid hydroxylations involved in the conversion of androstenedione to estrone. The first two reactions give rise to 19-hydroxy and 19-aldehyde structures, and the third, although still controversial, probably involves the C-19 methyl group with release of formic acid. In premenopausal women, the ovary is the most important site of aromatase and estrogen production. In postmenopausal women estrogen production takes place almost exclusively in extraglandular tissue. Androstenedione, produced primarily by the adrenal and, to a negligible extent, by the ovary, is converted to estrone by aromatase expressed in peripheral tissue such as adipose tissue23 and then subsequently converted to estradiol by 17-hydroxysteriod dehydrogenase. Through this pathway postmenopausal women produce approximately 100 mg of estrone per day, with higher levels observed in obese women. The adrenal gland secretes androstenedione (A), which enters plasma and then tissue. Extraglan- dular and breast tumor tissues contain the enzymes necessary to convert A to estrone (E1) and to estradiol (E2) or to estrone sulfate (E1S). These steroids circulate in the plasma at levels indicated within the brackets and are expressed as picogram per milliliter. Endocrine therapy for hormone receptor-positive breast cancer 717 with lower expression exhibited in the lung, brain, bone, and blood vessels. This binding interaction also involves a number of nuclear proteins, coregulators, as well as other components of the transcription machinery. Thus the genomic effects of estrogen are mainly the result of proteins synthesized from the regulation of transcription of a responsive gene. Carcinogenic effects the major concern with the use of synthetic estrogens either alone or as a part of the preparation of oral contraceptives has been the development of cancer. Studies that reported the link between the intake of diethylstilbestrol (a synthetic estrogen) during the first trimester of pregnancy and the incidence of clear cell vaginal and cervical adenocarcinoma in later life of the offspring exposed in utero established for the first time that developmental exposure to estrogens was associated with an increase in human cancer. Epidemiological studies have also linked high levels of natural estrogens to the development of cancer. Bone mineral density has also been shown to be a surrogate marker of estrogen exposure. A high endogenous estrogen concentration has been reported to be associated with greater bone mineral density in elderly women. However its reliability as a surrogate marker is controversial due to the low baseline levels observed among postmenopausal women and the timing of circulating estrogen level measurement with relation to the menstrual cycle being important in premenopausal women. Regardless, enough evidence exists connecting estrogens to the development of hormone-responsive breast cancer that has spawned a number of prevention trials that have used agents targeted either at blocking the production of estrogens or its interaction with its receptor. Selective estrogen receptor modulators and antiestrogens the first indication of the role of hormones in the development of breast cancer occurred more than a century ago when in 1896, Beatson observed that remission could be induced by removal of the ovaries in a subset of breast cancer patients. The second group of drugs blocks the production of estrogen by blocking the action of the aromatase enzyme and is known as aromatase inhibitors. Clinical pharmacology the high therapeutic index of tamoxifen has permitted wide variations in dosage with schedules and dosage of treatment varying depending on the country and its initial clinical trials that evaluated efficacy of this drug. Schedules of 10 mg twice daily or 20 mg once daily are recommended in the United States, although 10 mg three times daily and 20 mg twice daily have been used in other countries. As the potential for teratogenicity is unclear, it is as such not recommended in pregnant women. Furthermore tamoxifen is known to cause ovarian stimulation in premenopausal women with ovulatory cycles, and thus women taking tamoxifen who are at risk of getting pregnant should be counseled about various contraceptive options. Tamoxifen in the adjuvant setting Many randomized trials have addressed the question of tamoxifen efficacy in the adjuvant setting among women with early-stage breast cancer. In this report 1 year of tamoxifen conferred little benefit, 5 years of tamoxifen was significantly more effective than 2 years, with long-term follow-up still required for assessing the benefit of more than 5 years of adjuvant tamoxifen treatment. A number of prospective trials have looked at the efficacy of tamoxifen beyond 5 years in the adjuvant setting (Table 1). The investigators further reported that between years 5 and 14 of follow-up, 10 years of tamoxifen resulted in an absolute reduction in risk of breast cancer recurrence and mortality of 3. However, between years 5 and 14 of follow-up, there was also an increased cumulative risk of endometrial cancer among patients allocated to 10 years of tamoxifen (3. The investigators observed that continuing tamoxifen beyond 5 years reduced breast cancer recurrence beginning year 7 and breast cancer mortality after year 10. In view of the evidence from these two clinical trials, current guidelines recommend consideration of 10 years of adjuvant tamoxifen. Prevention of breast cancer Observations that long-term tamoxifen therapy reduced the incidence and risk of contralateral breast cancer in women with early-stage breast cancer fueled interest in exploring the effect of tamoxifen in preventing the occurrence of breast cancer. An overview of the main outcomes from the five main breast cancer prevention trials, covering more than 28,000 patients, has shown that tamoxifen produced a 38% reduction in breast cancer incidence (p < 0. Thus when considering using tamoxifen as a preventive agent, it will be important to balance the risks of developing complications to that of developing breast cancer, and as such the unrestricted use of tamoxifen among young women of reproductive age should be used with caution. The results of studies evaluating aromatase inhibitors as preventive agents will be discussed under the section titled "Aromatase inhibitors. Four large prospective trials have reported on the efficacy of raloxifene as a chemopreventive agent for breast cancer. In terms of side effects, compared to tamoxifen, raloxifene had fewer gynecological and thromboembolic events. Interestingly raloxifene reduced the risk of invasive breast cancer but had no effect on the incidence of ductal carcinoma in situ. In a prospective randomized clinical trial, 8556 postmenopausal women who had a bone mineral density T score of -2. However it was also reported to be associated with an increased risk of venous thromboembolic events. Toremifene Toremifene (Fareston) is a structural derivative of tamoxifen with similar antiestrogenic and estrogenic properties demonstrated in laboratory animals. In general, toremifene is highly protein bound, which could explain its long serum half-life. Toremifene is less potent than tamoxifen, and, consequently, clinical studies have evaluated doses of toremifene up to 240 mg/day. Toremifene is cross-resistant with tamoxifen, but clinical trials have shown that it exhibits a similar efficacy and side effect profile to tamoxifen and so may be used as an alternative to treat advanced breast cancer. A meta-analysis of several small studies investigating the use of trilostane in postmenopausal women with advanced breast cancer reported clinical benefit with this agent, and further trials are needed to evaluate its worth as an endocrine therapy for advanced breast cancer. In a preplanned subgroup analysis focusing on the cohort of patients with hormone receptor-positive disease, fulvestrant was observed to have a similar efficacy to tamoxifen. Based on these results fulvestrant is approved at a dose of 500 mg a month for the treatment of hormone receptor-positive metastatic breast cancer in postmenopausal who had progressed on prior antiestrogen therapy. As the 500 mg dosing schedule was found to be superior to the 250 mg dosing schedule, trials are underway investigating whether the use of the new approved dose of fulvestrant is superior to aromatase inhibitors in the treatment of advanced hormone receptor-positive breast cancer in the first-line setting. The most frequent side effects encountered are hot flashes, night sweats, and vaginal dryness similar to that seen in women undergoing menopause. Other less frequent but important side effects pertain to the bone, blood vessels, and carcinogenic effects.