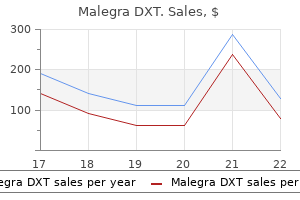
Order malegra dxt 130 mg line
A tendency exists for multinodular and diffusely infiltrative growth doctor's guide to erectile dysfunction malegra dxt 130 mg sale, and the true anatomic extent of these tumors, especially the superficial ones, is often underestimated, leading to significant risk of local relapse. The lowest-grade lesions have no capacity to metastasize but may become higher grade and hence acquire metastatic potential in a local recurrence. The high-grade lesions have an unusual but distinctive tendency to demonstrate lymph node metastasis, in addition to anticipated pulmonary and osseous spread. The principal features shared by tumors of all grades are hypocellular areas containing thin-walled curvilinear vessels, in which atypical, hyperchromatic small spindle and stellate cells with poorly defined, sometimes vacuolated cytoplasm are found. These small, bubbly vacuoles contain acid mucin rather than lipid ("pseudolipoblasts"). The cellularity and degree of pleomorphism in these lesions parallel the histologic grade. Rare tumors, usually of high grade, show remarkably epithelioid morphology, closely mimicking carcinoma or melanoma. The spindle and stellate cells have ultrastructural features of fibroblasts,328,332 or occasionally myofibroblasts, and this is reflected in the finding of focal actin positivity in some cases, particularly the higher-grade lesions with more solid areas. In high-grade lesions, areas of the tumor are indistinguishable from the nonspecific pattern of unclassified pleomorphic sarcoma (see earlier discussion), and, ultrastructurally, these bizarre cells may appear undifferentiated or "histiocyte-like. Intramuscular myxoma contains hardly any vessels at all and shows no nuclear atypia. A group of lesions also exists that is intermediate in appearance between intramuscular myxoma and low-grade myxofibrosarcoma, known as cellular myxoma (see later discussion). Superficial angiomyxoma does not show nuclear atypia or pleomorphism and often has an epithelial component. Myxoid variants of nerve sheath or smooth muscle tumors are generally less pleomorphic and most often show immunohistochemical evidence of their specific line of differentiation. Low-Grade Fibromyxoid Sarcoma Low-grade fibromyxoid sarcoma, first described by Evans,334,335 is both clinically and morphologically very different from the similarly named low-grade myxofibrosarcoma. It affects mainly younger adults in the third to fifth decades and arises at or below the level of fascia in a wide variety of soft tissue locations, but particularly around the lower limb girdle. Histologically, these are remarkably bland spindle cell neoplasms composed of uniform fibroblasts, most often arranged in a whorled pattern within a variably dense collagenous. Blood vessels may be arranged in thin-walled arcades in myxoid areas but are sparse in collagenous areas. Note the transition from low-grade tumor (left) to more pleomorphic malignant fibrous histiocytoma-like tissue. In terms of the most frequent differential diagnosis, low-grade myxofibrosarcoma is more vascular and more uniformly myxoid and shows greater nuclear hyperchromasia and pleomorphism. The important feature of this neoplasm is that, despite its banal morphology, as many as 30% of cases (possibly more) eventually metastasize and pursue a fatal clinical course over a period of 10 to 30 years. Hyalinizing spindle cell tumor with giant rosettes,337,344 a diagnostic term that has now fallen into disuse, is simply a morphologic variant of low-grade fibromyxoid sarcoma and differs only by the presence of strikingly hyalinized nodules surrounded in rosette-like fashion by more rounded to ovoid tumor cells in axial array. These tumors share the same cytogenetic abnormality as conventional low-grade fibromyxoid sarcoma342-344 and have exactly the same biologic potential. Sclerosing Epithelioid Fibrosarcoma Sclerosing epithelioid fibrosarcoma345-347 is very uncommon but increasingly recognized, affects mainly young to middle-aged adults of either sex, and presents as a deepseated mass on the limbs or trunk. At least 50% of patients have local recurrence and/or metastasis, but systemic spread is often delayed for 5 years or more. Histologically, these lesions consist of nests, cords, and strands of relatively small epithelioid cells, which usually have clear cytoplasm, set in an extensive, hyalinized collagenous stroma. Note the abrupt transition between collagenous and myxoid stroma, as well as the bland cytomorphology. Acral Myxoinflammatory Fibroblastic Sarcoma Acral myxoinflammatory fibroblastic sarcoma,348,349 also known as inflammatory myxohyaline tumor, occurs principally (but not exclusively) in the distal extremities, particularly the hands, of adults and presents as a slowly growing mass. It is characterized by frequent and repeated local recurrence, often necessitating some type of amputation, but metastasis appears to be very infrequent. Large, vacuolated pseudolipoblastic cells are a frequent feature in the myxoid areas. These tumors typically have infiltrative margins within subcutaneous or tenosynovial tissues. These pieces of evidence are now regarded widely as misjudged, and it is generally accepted that virtually none of the lesions in this category shows true histiocytic differentiation. Undoubtedly, the term fibrohistiocytic is a misnomer and falsely brings together a group of heterogeneous lesions, many of which are probably unrelated. However, the term is retained, at least for the time being, to facilitate a degree of diagnostic uniformity. Most such lesions can be more specifically classified, and the residuum is now regarded as unclassified or undifferentiated pleomorphic sarcomas (see later discussion in this chapter). Diffuse-type tenosynovial giant cell tumor, formerly known as pigmented villonodular tenosynovitis, is described in Chapter 25. It is now generally accepted to be a neoplastic (and even rarely metastasizing) lesion with distinctive molecular genetic aberrations, essentially identical to those in localized giant cell tumor. It is important to remember that lesions of this type may be entirely extra-articular and located well away from any synovial structure. Note the typical admixture of osteoclasts, mononuclear cells, and chronic inflammatory cells in a hyaline stroma. DeepBenignFibrousHistiocytoma Clinical Features A small proportion (<2%) of benign fibrous histiocytomas arise entirely within subcutaneous tissue or skeletal muscle, or within the abdominal cavity. Up to 25% of tumors recur locally, especially if they are only marginally or incompletely excised, and, as with cellular fibrous histiocytomas in skin, rare cases give rise to distant metastasis. The other principal difference from cutaneous lesions is that usually less cytologic polymorphism is seen: most cases consist largely of eosinophilic spindle cells with elongated or plump vesicular nuclei, arranged in a storiform pattern. Most cases present as a painless, slowly growing nodule, no more than 2 to 3 cm in diameter, and any aspect of any digit may be affected. After local excision, up to 10% of cases recur locally, but, in contrast to the diffuse type, which most often affects large joints (see Chapter 25), locally aggressive infiltration and invasion of bone do not occur. So-called malignant giant cell tumor of tendon sheath357,358 is more closely related to diffuse-type giant cell tumor of large joints and is also discussed in Chapter 25; comparable lesions involving the digits are very rare. Pathologic Features Localized giant cell tumor most often is a wellcircumscribed, lobulated mass with a variably yellow, tan, or whitish cut surface. It is composed of variable proportions of rounded eosinophilic mononuclear cells with vesicular nuclei, osteoclast-type multinucleate giant cells, foamy macrophages, siderophages, and chronic inflammatory cells. The stroma is collagenous and variably hyalinized; it often contains hemosiderin deposits and sometimes cholesterol clefts. The cellularity of these lesions is extremely variable; the more cellular cases often have few osteoclasts and may show a high mitotic rate, which frequently exceeds 10 mitoses per 10 hpf. In very hyalinized lesions the mononuclear cells may appear vacuolated and somewhat epithelioid, and osteoclasts may be sparse. The cleft-like spaces of the diffuse type of giant cell tumor (see Chapter 25) are generally absent. The mononuclear cells show close immunophenotypic similarities to normal synoviocytes,360 which are thought to be closely related to histiocytes. In addition, a common feature is the presence of large desmin-positive dendritic cells,360,361 probably comparable in nature to the so-called fibroblastic reticulum cells of lymph node. This wellcircumscribed subcutaneous lesion shows a monomorphic storiform appearance. This case shows branching pericytoma-like vessels and also contains numerous xanthoma cells. Mitoses are commonly present but usually number fewer than five per 10 hpf, and small foci of necrosis are sometimes found. A further pseudomalignant feature is the occasional finding of vascular invasion, as may rarely be seen in cutaneous lesions. With regard to the stroma, focal hyalinization or myxoid change may be seen, and branching hemangiopericytoma-like vessels are quite frequent. They are composed, in variable proportions, of fascicles of cytologically uniform, palely eosinophilic myofibroblasts (resembling fibromatosis) and nodular aggregates of histiocytoid cells, often admixed with osteoclastic giant cells. In cases with a scant histiocytoid component, the latter cells may be scattered singly or in only very small clusters within the myofibroblastic component.
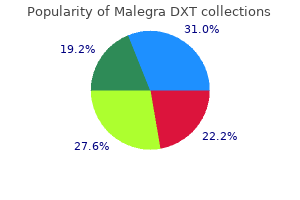
Best purchase malegra dxt
Report of a case (light and electron microscopic studies) and review of the literature erectile dysfunction rings 130mg malegra dxt sale. Fu Y S, Perzin K H 1977 Non-epithelial tumors of the nasal cavity, paranasal sinuses, and nasopharynx: a clinicopathologic study. Osseous and fibro-osseous lesions, including osteoma, fibrous dysplasia, ossifying fibroma, osteoblastoma, giant cell tumor and osteosarcoma. Waldron C A, Giansati J S 1973 Benign fibro-osseous lesions of the jaws: a clinico-pathologic-histologic review of sixty-five cases. Nevelle B W, Albenesius R J 1986 the prevalence of benign fibro-osseous lesions of the peridontal ligament origin in black women: a radiographic survey. Oda Y, Tsuneyoshi M, Shinohara N 1993 "Solid" variant of aneurysmal bone cyst (extragnathic giant cell reparative granuloma) in axial skeleton and long bones. Waldron C A, Shafer W G 1966 the central giant cell reparative granuloma of the jaws. Chakrabarty A, Mitchell P, Bridges L R 1998 Craniopharyngioma invading the nasal and paranasal spaces, and presenting as nasal obstruction. Br J Neurosurg 12: 361-363 154 Nasal Cavity, Paranasal Sinuses, and Nasopharynx 166. Jackson R T, Fitz-Hugh G S, Constable W C 1977 Malignant neoplasms of the nasal cavities and paranasal sinuses. Batsakis J G, Rice D H, Solomon A R 1980 the pathology of head and neck tumors: squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Pedersen E A, Hogetreit A C, Andersen A 1973 Cancer of the respiratory organs among workers at a nickel refinery in Norway. Trojussen W, Solberg L A, Hogetveit A C 1979 Histopathologic changes of nasal mucosa in nickel workers. Osborn D A 1970 Nature and behavior of transitional tumors in the upper respiratory tract. Crissman J D, Zarbo R J 1989 Dysplasia, in situ carcinoma, and progression to invasive squamous cell carcinoma of the upper aerodigestive tract. In: Harrison L B, Sessions R B, Waun K H (eds) Head and neck cancer: a multidisciplinary approach, 3rd ed. Pai S I, Westra W H 2009 Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Buhl R, Nabavi A, Fritsch M 2004 Nasopharyngeal extension of a craniopharyngioma in a 4 year old girl. Coppit G L 3rd, Perkins J A, Manning S 2000 Nasopharyngeal teratomas and dermoids: a review of the literature and case series. Ferlito A, Devaney K O 1995 Developmental lesions of the head and neck: terminology and biological behavior. Thompson L D, Miettinen M, Wenig B M 2003 Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Dardick I, Hammar S P, Sheithauer B W 1989 Ultrastructural spectrum of hemangiopericytoma: a comparative study of fetal, adult and neoplastic pericytes. Hansen T, Katenkamp K, Katenkamp D 2006 D2-40 staining in sinonasal-type hemangiopericytoma-further evidence of distinction from conventional hemangiopericytoma and solitary fibrous tumor. Kowalski P J, Paulino A F 2001 Proliferation index as a prognostic marker in hemangiopericytoma of the head and neck. Easton J M, Levine P H, Hyams V J 1981 Nasopharyngeal carcinoma in the United States. Dickson R I, Flores A D 1985 Nasopharyngeal carcinoma: an evaluation of 134 patients treated between 1971-1980. Batsakis J G, Solomon A R, Rice D H 1981 the pathology of head and neck tumors: carcinoma of the nasopharynx, part 11. Skinner D W, van Hasselt C A, Tsao S Y 1991 Nasopharyngeal carcinoma: a study of the modes of presentation. Buell P 1974 the effect of migration on the risk of nasopharyngeal cancer among Chinese. Vasef M A, Ferlito A, Weiss L M 1997 Nasopharyngeal carcinoma with emphasis on its relationship to Epstein-Barr virus. Laryngoscope 204: 99-102 Waghray M, Parhar R S, Taibah K 1992 Rearrangements of chromosome arm 3q in poorly differentiated nasopharyngeal carcinoma. Cancer Genet Cytogenet 140: 124-132 Shao J Y, Zeng W F, Zeng Y X 2002 Molecular genetic progression on nasopharyngeal carcinoma. Genes Chromos Cancer 30: 254-260 Lo K W, Huang D P 2002 Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin Cancer Biol 12: 451-462 Lo K W, Huang D P, Lau K M 1995 p16 gene alterations in nasopharyngeal carcinoma. Cancer Res 56: 2721-2725 Nicholls J M 1997 Nasopharyngeal carcinoma: classification and histologic appearances. Am J Otolaryngol 16: 103-108 Ahmad A, Stefani S 1986 Distant metastases of nasopharyngeal carcinoma: a study of 256 male patients. Herrold K M 1964 Induction of olfactory neuroepithelial tumors in Syrian hamsters by diethylnitrosamine. Whang-Peng J, Freier R E, Knutsen T 1987 Translocation t(11;22) in esthesioneuroblastoma. Nelson R S, Perlman E J, Askin F B 1995 Is esthesioneuroblastoma a peripheral neuroectodermal tumor Devaney K, Wenig B M, Abbondanzo S L 1996 Olfactory neuroblastoma and other round cell lesions of the sinonasal cavity. Luna M A 2009 the occult primary and metastatic tumors to and from the head and neck. Schiff B A, Mutyala S, Smith R S 2009 Metastatic cancer to the neck from an unknown primary site. An aggressive neoplasm derived from Schneiderian epithelium and distinct from olfactory neuroblastoma. Helliwell T R, Yeoh L H, Stell P M 1986 Anaplastic carcinoma of the nose and paranasal sinuses. Mills S E 2002 Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Mills S E, Fechner R E 1989 "Undifferentiated" neoplasms of the sinonasal tract: differential diagnosis based on clinical, light microscopic, immunohistochemical, and ultrastructural features. Kim B S, Vongtama R, Juillard G 2004 Sinonasal undifferentiated carcinoma: case series and literature review. Gallo O, Graziani P, Fini-Storchi O 1993 Undifferentiated carcinoma of the nose and paranasal sinuses. Vartanian R K 1996 Olfactory neuroblastoma: an immunohistochemical, ultrastructural and flow cytometric study. Long term follow-up and patterns of failure-the University of Virginia experience. Hyams V J, Batsakis J G, Michaels L 1988 Tumors of the upper respiratory tract and ear, 2nd ed. Barnes L 2001 Malignant melanoma of the nasal cavity and paranasal sinuses In: Barnes L (ed) Surgical pathology of the head and neck, 2nd ed. Wenig B M 1995 Laryngeal mucosal malignant melanoma: a clinicopathologic, immunohistochemical and ultrastructural study of four cases and a review of the literature. Thompson L D, Wieneke J A, Miettinen M 2003 Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Panje W R, Moran W J 1986 Melanoma of the upper aerodigestive tract: a review of 21 cases. Trapp T K, Fu Y S, Calcaterra T C 1987 Melanoma of the nasal and paranasal sinus mucosa. Franquemont D W, Mills S E 1991 Sinonasal malignant melanoma: a clinicopathologic and immunohistochemical study of 14 cases. Taira K 1985 Endocrine-like cells in the laryngeal mucosa of adult rabbits demonstrated by electron microscopy and by the Grimelius silver-impregnation method. J Cutan Pathol 9: 61-81 Barnes L 1986 Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses.
Purchase discount malegra dxt on line
Dysplastic gangliocytomas are histologically characterized by hypertrophic cerebellar folia because of proliferation of abnormal hypertrophic ganglion and neuronal cells superficially resembling Purkinje cells erectile dysfunction 60 year old man buy cheap malegra dxt on-line. The treatment of dysplastic gangliocytoma of the cerebellum is surgical resection. In the series of Abel and colleagues,355 31% of patients with long-term follow-up underwent at least one additional resection for recurrent disease. Silver impregnation demonstrates abnormally shaped and oriented neuritic processes arising from these neoplastic neurons. Often reduction is seen of the adjacent granular layer and demyelination of the cortical white matter. Studies trying to correlate the expression of synaptic and surface membrane proteins related to Purkinje cells and/or granular cell neurons show conflicting results. Dysplastic gangliocytomas of the cerebellum are linked to Cowden disease, an autosomal dominant disorder characterized by multiple hamartomas and neoplasms. Central neurocytomas are supratentorial, intraventricular neuronal tumors that appear to have a favorable prognosis after surgical resection. In a series of 10 cases studied at the University of Virginia, the age at surgery ranged from 6 to 52 years (mean 24 years),364 which corresponds to that in other reported series. The histologic features of central neurocytomas are typical and relatively constant. The tumors are composed of a uniform population of cells with round to slightly lobulated nuclei and finely speckled chromatin in a conspicuously fibrillary matrix. Delicate microvasculature forms a branching network in a pattern slightly reminiscent of oligodendrogliomas. The uniform cells, the finely fibrillated matrix, and the typical microvessels are readily appreciated on smear preparations. A consistent and characteristic feature of these tumors is anuclear islands composed of dense fibrillary matrix. Smear preparations of a central neurocytoma show uniform cells with round nuclei and delicately dispersed chromatin. The cytoplasm of the cells is ill defined, and the eosinophilic matrix is moderately fibrillary. The appearance of central neurocytoma in tissue sections typically consists of homogeneous cell populations partially delineated by mild proliferation of a delicate vascular stroma. Higher magnification of a routine preparation of central neurocytoma demonstrates the diffusely dispersed and delicate chromatin pattern in the uniform round nuclei. Synaptophysin immunoreactivity, consistent with the neurocytic nature of the tumors, is diffusely distributed within the tumor but is more prominent in the anuclear zones. Microtubule-associated protein-2 immunohistochemistry demonstrates the intense immunoreactivity of the densely packed neuritic processes. Cerebellar liponeurocytomas are very rare tumors arising in the cerebellum of adults that are histologically characterized by neuronal differentiation with focal lipidization. They arise in an older population than patients with central neurocytoma, most occurring in the fifth and sixth decades of life (mean age at presentation 50 years). A few cases with similar histopathologic features but located in the supratentorial region have also been reported. Cerebellar liponeurocytomas are characterized by a biphasic pattern of relatively uniform round cells resembling that of neurocytoma, although intermingled with a varying proportion of lipidized cells. Mitoses are infrequent, and microvascular proliferation and/or necrosis is inconspicuous or absent. The adipose component may also be labeled by neuronal and glial markers indicating that fatcontaining cells represent lipomatous differentiation of tumor cells rather than entrapped adipocytes. Astrocytic cell processes, packed with intermediate filaments, are present but not conspicuous. The histogenesis of cerebellar liponeurocytoma and its relationship to other neuronal tumors is not well understood. It appears that cerebellar liponeurocytomas are a pathologically and genetically distinct neuronal and glioneuronal tumor. However, tumor recurrence has been reported in several instances, mainly in those cases that were subtotally resected. Although these features tend to be associated with a less-favorable outcome, particularly recurrence, they are less well correlated with prognosis than in gliomas,362,365 and extent of surgery is the best prognostic parameter. However, in cases of incomplete or subtotal resection, conventional external beam radiation therapy or focused radiosurgery has been advocated. Tumors with similar histologic features as central neurocytomas have been described in extraventricular locations. These tumors share histologic features with central neurocytomas but exhibit a wider morphologic spectrum with regard to cellularity and proliferation rates. However, subtotal resection, atypical histologic features, and high cell proliferation rates appear to correlate with recurrence. Although cerebellar liponeurocytomas appear to have a favorable prognosis, recurrence is seen in nearly two thirds of cases. Mixed Neuronal-Glial Tumors Overview Mixed neuronal-glial tumors (see Table 26-4) are more common than the purely neuronal tumors discussed earlier. Many of these tumors are associated with seizure disorders, particularly gangliogliomas. Most of these tumors are low grade, and their recognition is important to avoid their misidentification as ordinary gliomas and to prevent unnecessary adjuvant treatment. The majority of tumors arise in the first three decades of life (median 26 years; range 2-65 years) with no significant sex preference. Grossly, gangliogliomas are relatively well demarcated and characteristically exhibit a cyst-mural nodule architecture. Histologically, gangliogliomas are composed of a mixture of neoplastic ganglion cells and glial cells. The ganglion cells are dysmorphic, are abnormal in shape, and may show considerable variation in size and shape. Bizarre and binucleate forms are present, but the latter may be rare in some tumors. Smears of ganglioglioma readily demonstrate mixed astrocytic and ganglionic populations in a fibrillary stroma. Eosinophilic granular or hyaline bodies are commonly intermixed within the glial component. In tissue sections, the mixed population of neoplastic astrocytes and abnormal ganglion cells is typically embedded in a fibrovascular stroma. In this temporal lobe ganglioglioma, the astrocytic component resembles a pilocytic astrocytoma. Bielschowsky silver impregnation readily demonstrates the abnormally orientated and shaped leashes of neuritic processes from the ganglion cells. The stromal component is variable and ranges from delicate fibrovascular elements admixed with an abundant glial population to a relatively intense desmoplastic reaction that nearly obscures the glial matrix. On occasion, glomeruloid capillaries and/or hyalinized vessels are sufficiently abundant as to mimic a vascular malformation. The perivascular lymphocytic infiltration should not be mistaken for neuroblastic cells. Ki67 labeling index is low in the great majority of gangliogliomas, and it appears to be confined to the glial component. A, the glial component of this anaplastic ganglioglioma is composed of small cells with scant glial cytoplasm and hyperchromatic nuclei. B, In the same tumor, ganglion cells were readily identified by silver impregnation stain. Clinically, anaplastic transformation should be suspected in patients with a recent exacerbation of chronic seizure activity and increasing T2 signal intensity and contrast enhancement with neuroimaging. Desmoplastic Infantile Ganglioglioma and Desmoplastic Cerebral Astrocytoma of Infancy, World Health Organization Grade I Clinical Features. The tumors typically present as a very large supratentorial tumor, with solid and cystic components. The majority of tumors manifest clinical symptoms between 2 and 24 months of age (mean 6 months; median 4 months) with a slight male predominance. The frontoparietal regions are most commonly involved, but the tumors may also arise in other hemispheric sites; combined sites are quite common. Confinement to deep structures or communication with the ventricular system is rare and has been described in only very occasional cases. Reticulin deposition is a notable feature of these neoplasms, which corresponds, in part, to the production of basal lamina by the neoplastic astrocytes.
Purchase generic malegra dxt pills
Fantone J C erectile dysfunction treatment options-pumps buy cheapest malegra dxt, Geisinger K, Appelman H D 1982 Papillary adenoma of the lung with lamellar and electron dense granules. Suster S, Moran C A 1993 Pulmonary adenofibroma: report of two cases of an unusual type of hamartomatous lesion of the lung. Gaffey M J, Mills S F, Askin F G 1988 Minute pulmonary meningothelial-like nodules. Suster S, Moran C A 1995 Unusual manifestations of metastatic tumors of the lungs. Henderson D W, Shilkin K B, Whitaker D 1998 Reactive mesothelial hyperplasia vs mesothelioma, including mesothelioma in situ. Ordonez N G 2005 D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Mayall F G, Goddard H, Gibbs A R 1992 Intermediate filament expression in mesotheliomas: leiomyoid mesotheliomas are not uncommon. Clover J, Oates J, Edwards C 1997 Anticytokeratin 5/6: a positive marker for epithelial mesothelioma. Suster S, Moran C A 2006 Applications and limitations of immunohistiochemistry in the diagnosis of malignant mesothelioma. Harwood T R, Gracey D R, Yokoo H 1976 Pseudomesotheliomatous carcinoma of the lung. Attanoos R L, Gibbs A R 2003 "Pseudomesotheliomatous" carcinomas of the pleura: a 10-year analysis of cases from the Environmental Lung Disease Research Group, Cardiff. Scharifker D, Kaneko M 1979 Localized fibrous "mesothelioma" of pleura (submesothelial fibroma): a clinicopathologic study of 18 cases. Burrig K-F, Kastendieck H 1984 Ultrastructural observations on the histogenesis of localized fibrous tumors of the pleura (benign mesothelioma). England D M, Hochholzer L, McCarthy M J 1989 Localized benign and malignant fibrous tumors of the pleura: a clinicopathologic review of 223 cases. Briselli M, Mark E J, Dickersin R 1981 Solitary fibrous tumor of the pleura: eight new cases and review of 360 cases in the literature. Moran C A, Suster S, Koss M N 1995 Smooth muscle tumors presenting as pleural neoplasms. Moran C A, Suster S 2003 Primary mucoepidermoid carcinoma of the pleura: a clinicopathologic study of three cases. Hedlung G, Bisset G, Bove K 1989 Malignant neoplasms arising in cystic hamartomas of the lung in childhood. These are similar to tumors of stratified squamous epithelium occurring at other body sites and only infrequently present problems in diagnosis. The mesenchymal tissues of the mouth are less frequently the source of neoplasms, but several overgrowths occur in response to chronic irritation, and it is important that these are distinguished from true neoplasms. Tumors of the jaws may also be problematic because many are rare or may show features specific to this site. Odontogenic cysts and tumors are derived from the dental tissues and show variable features that may recapitulate stages of tooth development. Odontogenic tumors are rare but often pose diagnostic problems for the pathologist unfamiliar with dental tissues; hence they receive more emphasis in this chapter. Odontogenic cysts are common and, although rarely neoplastic, are included because classification may be confusing and they frequently cause diagnostic difficulty. Histologic Appearances In the typical squamous cell papilloma the epithelium is of fairly uniform thickness, with variable keratinization or hyperkeratosis, and covers cores of loose vascular connective tissue. Some cytologic atypia may be present in the deeper cell layers, and increased mitotic activity is often present. This may be accentuated if infestation by Candida albicans occurs in the superficial layers of the epithelium. The typical histologic appearances of verruca vulgaris are seen in some oral lesions, and a clinical association with cutaneous warts on the fingers may exist. Condyloma acuminatum, also reported in the mouth, cannot be diagnosed with certainty on histologic grounds. Several variants exist, although these overlap in clinical and histologic appearance. All are slow growing, and no evidence is available that these lesions are premalignant. Histologically fibrous overgrowth covered by irregularly hyperplastic epithelium is seen. Arrested, fragmented metaphases, described as mitosoid cells, may be present in the stratum spinosum. Squamous Cell Carcinoma Clinical Features Squamous cell carcinoma3 is much the most common malignant neoplasm of the mouth. Most 6 Tumors of the Oral Cavity 247 Histologic Appearances Oral squamous cell carcinomas show the same range of features and variants as such carcinomas at other sites, and criteria for grading of tumors is the same (see Chapter 23). Diagnosis is usually straightforward, although the earliest stages of invasion may be difficult to determine, and criteria for microinvasion are not adequately defined. Fortunately, such lesions are unlikely to metastasize at the stage when the term microinvasive might be used, and the local excision appropriate for a verrucous lesion is also the appropriate treatment for microinvasive carcinoma. This is often associated with obviously increased mitotic activity, and it is important to recognize that this is disproportionate to the degree of dysplasia. It is often wise to defer a diagnosis of malignancy in such cases until fungal infection is treated and biopsy of the lesion is repeated. Many attempts have been made to devise schemes for predicting the prognosis of oral carcinomas from analysis of histologic features,7,8 but none of these has found general favor. A tumor-invasive front composed of single cells or small cords is used by some pathologists as a predictor of increased likelihood of metastatic spread to lymph nodes. This should be measured from the actual surface for flat lesions or the presumed original epithelial surface for exophytic or ulcerated lesions. Tumor thickness exceeding 5 mm is associated with a significantly increased risk of metastasis. Some tumors are composed entirely of the variant type, but in many instances lesions show a mixture of conventional squamous cell carcinoma and other patterns. In these cases it is probably best to report the mixed patterns and advise that the behavior is likely to be determined by the most aggressive variant present. Spindle cell squamous carcinoma is a pleomorphic spindle cell neoplasm most frequently found in the head and neck. In any such case, spindle cell carcinoma should always be included in the differential diagnosis. In the larynx, spindle cell carcinomas are often polypoid, but in the oral cavity they are similar to conventional carcinomas and present as ulcers or red lesions. The next most frequent site is the "soft palate complex" consisting of the soft palate, anterior pillar of fauces, and retromolar area. In parts of the world with a high incidence of oral cancer, such as India, most oral cancers arise in areas of leukoplakia. By contrast, in developed countries, small intraoral carcinomas are almost always red or predominantly red. In the majority of cases, it is probably wise to insist on finding areas of conventional squamous cell carcinoma or severe epithelial dysplasia, usually at the growing edge of the tumor, before making the diagnosis of spindle cell carcinoma, although mucosal ulceration may preclude this. The term adenoid squamous cell carcinoma describes cases in which loss of intercellular adhesion in the center of tumor islands resembles glandular differentiation. In these lesions involvement of salivary gland ducts is seen, often concurrently with tumor arising from the surface mucosa. The main differential diagnosis is from mucoepidermoid carcinoma and is made by the recognition of discrete areas of adenocarcinoma and of squamous cell carcinoma arising from the surface epithelium. A small number of otherwise conventional squamous cell carcinomas may show a few mucus-secreting cells, and demonstration of these, in the absence of ductal differentiation. It is also important to recognize correctly the potentially confusing presence of invasion by squamous cell carcinoma into normal salivary gland. Note that the acinar tissue and the ducts are from existing gland and not part of the tumor, although one duct shows squamous metaplasia. The basaloid areas show demarcated nests of cells with peripheral palisading, basal cell morphology, and numerous mitotic figures. They are most typically seen in the posterior aspect or base of the tongue and must be distinguished from other basaloid lesions, particularly solid adenoid cystic carcinoma or salivary duct carcinoma. This term was first coined for exophytic, massively acanthotic lesions showing minimal cytologic atypia that would not, on strict histologic grounds, be diagnosed as carcinoma.
Order malegra dxt 130mg visa
Rorke L B erectile dysfunction pills not working buy generic malegra dxt 130 mg on-line, Packer R, Biegel J A 1996 Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. Broughton W L, Zimmerman L E 1978 A clinico-pathologic study of 56 cases of intraocular medulloepitheliomas. Caccamo D V, Herman M M, Rubinstein L J 1989 An immunohistochemical study of the primitive and maturing elements of human cerebral medulloepitheliomas. Khoddami M, Becker L E 1997 Immunohistochemistry of medulloepithelioma and neural tube. Troost D, Jansen G H, Dingemans K P 1990 Cerebral medulloepithelioma-electron microscopy and immunohistochemistry. Dehner L P, Abenoza P, Sibley R K 1988 Primary cerebral neuroectodermal tumors: neuroblastoma, differentiated neuroblastoma, and composite neuroectodermal tumor. Thomas G A, Raffel C 1991 Loss of heterozygosity on 6q, 16q, and 17p in human central nervous system primitive neuroectodermal tumors. Schnitzer J 1988 Astrocytes in the guinea pig, horse and monkey retina: their occurrence coincides with the presence of blood vessels. A clinicopathologic study of 18 cases with special reference to the issue of metaplasia. An immunohistochemical, histochemical and ultrastructural study-with review of literature. Mawrin C, Perry A 2010 Pathological classification and molecular genetics of meningiomas. Couce M E, Aker F V, Scheithauer B W 2000 Chordoid meningioma: a clinicopathologic study of 42 cases. Chen W Y, Liu H C 1990 Atypical (anaplastic) meningioma: relationship between histologic features and recurrence-a clinicopathologic study. Schnitt S J, Vogel H 1986 Meningiomas: diagnostic value of immunoperoxidase staining for epithelial membrane antigen. Artlich A, Schmidt D 1990 Immunohistochemical profile of meningiomas and their histological subtypes. Ng H K, Wong A T 1993 Expression of epithelial and extracellular matrix protein markers in meningiomas. Hahn H P, Bundock E A, Hornick J L 2006 Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics. Rajaram V, Brat D J, Perry A 2004 Anaplastic meningioma versus meningeal hemangiopericytoma: immunohistochemical and genetic markers. Hsu D W, Efird J T, Hedley-Whyte E T 1997 Progesterone and estrogen receptors in meningiomas: prognostic considerations. Weber R G, Bostrom J, Wolter M 1997 Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Riemenschneider M J, Perry A, Reifenberger G 2006 Histological classification and molecular genetics of meningiomas. A populationbased study of incidence, clinicopathological features and outcome in Alberta from 1975 to 1996. Perry A, Scheithauer B W, Nascimento A G 1997 the immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Liubinas S V, Maartens N, Drummond K J 2010 Primary melanocytic neoplasms of the central nervous system. Nonaka D, Rodriguez J, Rosai J 2007 Extraneural hemangioblastoma: a report of 5 cases. Hoang M P, Amirkhan R H 2003 Inhibin alpha distinguishes hemangioblastoma from clear cell renal cell carcinoma. Da Silva A N, Lopes M B, Schiff D 2006 Rare pathological variants and presentations of primary central nervous system lymphomas. Morgello S 1995 Pathogenesis and classification of primary central nervous system lymphoma: an update. Glenn O A, Barkovich A J 1996 Intracranial germ cell tumors: a comprehensive review of proposed embryologic derivation. Jennings M T, Gelman R, Hochberg F 1985 Intracranial germcell tumors: natural history and pathogenesis. Kirkove C S, Brown A P, Symon L 1991 Successful treatment of a pineal endodermal sinus tumor. Sano K, Matsutani M, Seto T 1989 So-called intracranial germ cell tumours: personal experiences and a theory of their pathogenesis. Atkin N B, Baker M C 1983 I(12p): specific chromosomal marker in seminoma and malignant teratoma of the testis. Yaargil M G, Abernathey C D, Sarioglu A C 1989 Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. With regard to those lesions that truly recapitulate some form of nerve sheath differentiation, these remain a very heterogeneous and complex group with variably precise diagnostic criteria. This reflects the sophisticated and intricate structure of peripheral nerves, which comprise myelinated or unmyelinated axons embedded in an endoneurial matrix composed of Schwann cells and fibroblasts, in turn surrounded by a layer of perineurial cells; such nerve "fascicles" are then bound together within the fibroblastic connective tissue known as epineurium to form a peripheral nerve. The range of structure and cell types from which so-called nerve sheath tumors may either arise, or differentiate toward, is therefore quite broad. A clinically distinctive subgroup in the surgical category presents with right upper quadrant abdominal pain, or even jaundice, after previous cholecystectomy. Histologically, traumatic neuroma consists of a poorly circumscribed and disorderly outgrowth of all the normal components of a nerve fascicle, that is, fibroblasts, Schwann cells, perineurial cells, and numerous small nerve fibers. This proliferation generally takes place within collagenous fibrous tissue; on occasion the latter may become inflamed or myxoid, depending on external factors. Morton Neuroma (Morton Metatarsalgia) Morton neuroma is included in a book on tumors only because it may be identified clinically as a localized swelling. Morton neuroma3 presents with severe, lancinating pain in the sole of the foot, usually in the region of the metatarsal heads or metatarsophalangeal joints. The use of footwear that necessitates extension of the foot at the metatarsophalangeal joints- such as high-heeled shoes-is probably contributory. At operation, the surgeon finds thickening or fusiform swelling of one or more of the digital plantar nerves, often with thickening of adjacent tenosynovial tissues. Histologically, the lesion is characterized by marked endoneurial, perineurial, and epineurial fibrosis and hyalinization associated with loss of axons4. In addition, fibrosis and thickening of adjacent vessel walls are typically also seen, as well as fibrosis within adipose tissue. The two principal causes are trauma, either lacerating or penetrating, and surgery, especially limb amputation. The former occurs most often in young adults, whereas the latter is most common in the elderly and parallels the incidence of limb ischemia due to peripheral vascular disease. Pacinian Neuroma Pacinian neuroma is an uncommon but distinctive type of neuroma that typically arises on the fingers, usually in adulthood, at the site of previous injury. Almost all reported examples have arisen after local trauma, and these neuromas are therefore regarded as reactive rather than neoplastic. Histologically, digital pacinian neuroma consists simply of an unencapsulated hyperplastic collection of normal-sized pacinian corpuscles. Lipomatosis of Nerve (Fibrolipomatous Hamartoma; Neural Fibrolipoma) Lipomatosis of nerve is a rare condition that, although it has been the subject of many single case reports, has only once been documented properly in a large series. The onset is usually in early childhood with presentation to a clinician in early adulthood. The sex incidence is equal, and up to 25% of patients have associated macrodactyly due to lipohypertrophy. Exceptional cases may occur outside the region of the wrist or forearm, usually in a more proximal location. Inadvertent excision (or attempted excision) of the fusiform mass inevitably leads to a significant neurologic deficit. Histologically, the epineurium of the affected nerve is expanded by copious, poorly circumscribed, mature fibrofatty tissue.
Bitterbark (Fever Bark). Malegra DXT.
- How does Fever Bark work?
- What is Fever Bark?
- Are there safety concerns?
- Are there any interactions with medications?
- Dosing considerations for Fever Bark.
- Fever, hypertension, diarrhea, malaria, and arthritis-like pain (rheumatism).
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96452
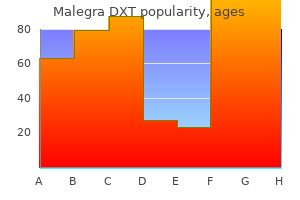
Discount malegra dxt master card
Melanin pigmentation is often scanty and sometimes difficult to find in regular H&E-stained sections treatment for erectile dysfunction before viagra order malegra dxt 130mg free shipping. S-100 protein is positive in almost all lesions, but melan A is focally present in the intraepidermal and superficial dermal components but usually negative in the deeper areas in most cases. The term desmoplastic melanoma should be used only for neoplasms with uniform cytologic features. Importantly, a small but significant subset of these lesions lack any evident epidermal or junctional component. High-grade, cytologically pleomorphic lesions should be referred to as pleomorphic spindle cell melanomas, because they probably carry a poorer prognosis. The differential diagnosis of the latter includes spindle cell carcinoma, atypical fibroxanthoma, and malignant peripheral nerve sheath tumor. The neurotropic melanoma variant1005-1011 is closely related to (if not the same as) desmoplastic melanoma; frequently, combined patterns exist. They also tend to arise in similar clinical settings, especially around the head and neck. Histologically, the neoplastic cells are arranged in a fascicular growth pattern reminiscent of that of a peripheral nerve sheath tumor. The neural involvement can be subtle; sometimes a rare enlarged or dysmorphic nucleus is the only clue to the correct diagnosis. The differential diagnosis consists of a variety of benign neural and melanocytic lesions, including desmoplastic melanocytic nevus, neurofibroma, and malignant schwannoma. The balloon cell type of melanoma1012-1015 is characterized by large melanocytes with abundant clear cytoplasm. These lesions usually display little cytologic pleomorphism and negligible numbers of mitoses, which may lead to misdiagnosis as balloon cell melanocytic nevus. When the lesion is also amelanotic, the differential diagnosis includes xanthoma, clear cell adnexal neoplasms, and metastatic disease, most notably, from a renal cell carcinoma. The signet ring cell melanoma1016-1018 is a cytologic variant in which the tumor cells have large eosinophilic to clear cytoplasm with eccentric nuclei. A serious potential exists to confuse this form of melanoma with other neoplasms that have signet ring cell cytology, principally mucin-producing adenocarcinomas. Cytologically, the lesion is similar to spindle squamous carcinoma and atypical fibroxanthoma. It is sometimes possible to find adjacent areas of blue nevus in well-studied specimens, suggesting malignant transformation, at least for some lesions. A relatively small subset of malignant melanomas (although the proportion is higher in metastatic lesions, perhaps as many as 10%) are characterized predominantly by rhabdoid features,1020-1022 manifest as rounded epithelioid cytomorphology with hyaline eosinophilic cytoplasmic inclusions and vesicular nuclei with macronuclei. This pattern, although further confirming the nonspecificity of rhabdoid morphology in extrarenal neoplasms (see Chapter 24), can cause diagnostic problems because the rhabdoid component may be immunonegative for S-100 protein and melan A, thus necessitating a careful search for either nonrhabdoid areas or else a junctional or intraepidermal component. Some tumors composed of cells usually not thought to be primarily dermal, such as those with cells of myeloid or lymphoid phenotype, may also have a prominent dermal component and are included in this section. Tumors of Dermal Stroma Fibrous Histiocytoma (Dermatofibroma) Fibrous histiocytoma1023-1028 is a spectrum of benign, welldemarcated dermal tumors composed of mononuclear and/or multinucleate cells with phagocytic or myofibroblastic attributes and with a variety of growth patterns. The cause of fibrous histiocytoma is unknown,1026 and no convincing physical evidence exists associating it with insect bites1029 or other trauma, although many authors have theorized such an association. Cytogenetic data have shown that at least some of these lesions are clonal,1030 arguing in favor of a neoplastic process. Clinically, fibrous histiocytomas typically occur in adults, but children may be affected. Most measure between 5 mm and 2 cm in diameter and are characterized by a range of skin-colored, to brown, to purple smooth plaques, nodules, or polyps that are surfaced by intact skin, which is commonly thickened. Fibrous histiocytomas of any type may be strongly suspected by pinching the nodule between the fingers and observing that the tumor is fixed within the dermis. Only the more cellular, aneurysmal, or deeply infiltrative cases persist or recur with any significant frequency. Histologically, fibrous histiocytomas are a range of lesions that are usually symmetric and principally dermal, with relatively circumscribed but unencapsulated borders. The histologic patterns range from diffuse, to reticular, to hemangioma-like, to keloidlike. The classic attribute, however, is the storiform, pinwheel, or curlicue pattern, which is observed in most cases. Cytologically, the lesions are composed of variably ovoid, to fusiform mononuclear, to multinucleate cells with amphophilic cytoplasm. Foamy macrophages and giant cells, including Touton cells, are observed in some cases. If a fibrous histiocytoma is polarized, birefringent collagen is observed within the lesion, in contrast to dermatofibrosarcoma protuberans. Ultrastructurally, the lesions may have cytologic features of fibroblasts and lipid-laden histiocytes. These are symmetric epidermal hyperplasia over the lesion, a storiform growth pattern, and hyalinized collagen fibers that are surrounded (or entrapped) by spindle cells at the peripheral border of the lesion (keloidization of collagen). Individual lesions of fibrous histiocytoma are characterized by mixtures of architectural and cytologic patterns or may be predominantly of one type. When the latter is the case, specific names have been offered, as discussed in the following section. One example that tends to arise mainly in young adults and may show rapid growth is aneurysmal fibrous histiocytoma. Histologically, numerous bloodfilled cavernous cavities without endothelial lining are present within the background of a cellular hemosiderotic fibrous histiocytoma (see later discussion). Prominent hemosiderin deposition, numerous siderophages and giant cells, and moderate numbers of cells in mitosis are often seen. Some degree of cellular polymorphism is usually present, along with hyalinized collagen bundles surrounded by tumor cells in the periphery of the lesion. Understandably, aneurysmal fibrous histiocytoma has, at times, some similarity to the nodular stage of Kaposi sarcoma. The differential diagnosis also includes angiomatoid malignant fibrous histiocytoma (see Chapter 24), spindle cell hemangioma (see Chapter 3), and spindle cell melanoma. Atypical Fibrous Histiocytoma, Dermatofibroma with Monster Cells, Pleomorphic Fibroma, and "Pseudosarcomatous" Fibrous Histiocytoma. These terms1049-1054 are used to describe a clinically nondistinctive variant that contains pleomorphic cells in an otherwise typical histologic background of fibrous histiocytoma. Such cytologic pleomorphism is characterized by bizarre hyperchromatic giant cells and mononuclear cells with large vesicular nuclei. The epithelioid fibrous histiocytoma1055-1059 is a notable variant with a peculiar epithelioid cytomorphology. The neoplastic cells have ample eosinophilic cytoplasm with vesicular nuclei, thus resembling epithelioid cell nevi (Spitz), and some cells are binucleate. These usually exophytic lesions have no junctional component and are not organized in theques. The (hyper) cellular type of fibrous histiocytoma1032,1034,1035,1060 accounts for approximately 5% of cases and, by virtue of its relative monomorphism and fascicularity. Extension into superficial subcutis and small areas of central necrosis are relatively frequent. Other reported variants of fibrous histiocytoma include clear cell1061,1062 and granular cell1063-1065 dermatofibroma, deep penetrating dermatofibroma,1066 cholesterotic fibrous histiocytoma,1037 lipidized fibrous histiocytoma,1038 fibrous histiocytoma associated with (collision It is probable, however, that the many morphologic variants are phases that might be observed in any given fibrous histiocytoma, evidenced by the fact that mixed lesions are often encountered. In this regard, sclerosing hemangioma, hemosiderotic fibrous histiocytoma, and aneurysmal fibrous histiocytoma represent a continuous spectrum. The differential diagnosis includes scar, keloid, sclerotic fibroma,1077-1079 some hemangiomas, giant cell fibroblastoma, dermal nodular fasciitis,1080 fat-storing hamartoma of dermal dendrocytes,1081 dermatomyofibroma,1082 atypical fibroxanthoma, and dermatofibrosarcoma protuberans. Giant Cell Fibroblastoma Giant cell fibroblastoma1085-1087 is a biologically low-grade dermal or subcutaneous tumor composed of fusiform cells, multinucleate cells, and so-called angiectoid spaces lined by giant cells. Typical locations are the trunk and, less often, the lower extremities,1088 but other sites have been recorded.
Syndromes
- Have difficulty breathing
- Coma
- The use of iodized salt in many food products in the United States today prevents a lack of iodine in the diet.
- Walks up stairs while holding on with one hand
- Lethargy
- Quinine
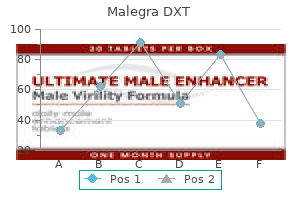
Purchase malegra dxt on line
Peripherally the tumor has a low-grade appearance with large vascular channels lined by endothelial cells new erectile dysfunction drugs 2013 buy generic malegra dxt line. The predominant pattern is, however, low grade, particularly at the periphery of the tumor. This variation in pattern further emphasizes the need to sample tumors adequately. Morphologically highgrade tumors are composed predominantly of solid tumor with a paucity of distinct vascular channels. In these tumors distinction from other spindle cell neoplasms is the main problem. Conservative surgery with radiotherapy is increasingly used in the treatment of breast cancer, and it is estimated that about 0. The interval between radiation exposure and develop ment of angiosarcoma is usually between 2 and 10 years. Angiosarcoma arising after radiotherapy is more common than de novo angiosar coma, and, because it is associated with breast cancer treatment, it is seen in older women. Atypical vascular lesions can also arise after radiotherapy and must be dis tinguished from welldifferentiated angiosarcoma. The tumor has a very aggressive behavior, spreading rapidly through the area of lymphedema and disseminating widely throughout the body. Histologically the tumor is composed of papillary or solid growth of poorly differentiated spindle cell or epithelioid cells. This morphology led to speculation that these tumors may represent retrograde vascular spread of breast carcinoma cells rather than true angiosarcoma. Immunohistologic and ultrastructural studies, however, confirmed that they are vascular in origin769,770 and best regarded as veryhighgrade lymphangiosarcoma. An invasive pure spindle cell tumor with a mesenchymal phenotype (vimentin positivity only). Clin ically the tumor presents with a short history of a mass, which on examination is firm and freely mobile. These lesions are rounded and well circumscribed and have a vague multinodular cut surface appearance. These tumors are circumscribed and have a false capsule of compressed breast tissue, with only rare entrapped mammary parenchymal structures. They are composed of spindle cells often arranged in short bundles within a collagenous stroma. The nuclei are oval or stubby with slightly irregular outline, small nucleoli, and occasional grooves. Ultrastructurally the tumor cells contain bundles of myofilaments with focal densities. Other Sarcomas Most types of sarcoma have been described at least rarely in the breast. The diagnostic criteria for tumors not spe cific to the breast are covered in Chapter 24. It must be borne in mind that the breast is a rare site for primary presentation of sarcoma. Clusters of plump spindleshaped cells separated by bands of hyaline collagen are the two characteristic features of this tumor. However, solitary fibrous tumor is more patternless; has coarser, thick bands of collagen; and is usually negative for desmin and smooth muscle actin. Furthermore, mammary myofibroblastoma shares a distinct 13q chromosome rearrangement with spindle cell lipoma and cellular angiofibroma. The tumors usually present as a breast mass that on examination is firm and circumscribed or illdefined. Mammography may show a spiculated mass indistinguishable from breast cancer, but results can be normal. A, An ill-defined infiltrative tumor that surrounds preexisting mammary structures. Excision margins should be examined closely, but it is increasingly appreciated that a good correlation does not exist between margin status and recurrence. Aggressive variants are described that may extend through the breast into the chest wall and mediastinal tissues. However, static disease and even complete resolution without treatment are described. Fibromatosis of the breast is similar to deep desmoidtype fibromatosis arising else where (see Chapter 24). The diagnosis is based on rec ognition of a bland infiltrative spindle cell proliferation. The tumor extends along fibrous septae, and histologic evidence of the disease may be present a con siderable distance from the macroscopically visible focus of tumor. The cellularity may vary, and collagenous or hyalinized areas may be present, often centrally. Mammary parenchymal ducts and lobules, when surrounded by fibromatosis, may exhibit features of gynecomastoid hyperplasia with micropapillary epithelial proliferation. Nuclear expression of catenin is present in about 80% of fibromatoses of the breast and is also described in fibroadenomas, phyllodes tumors, and spindle cell carcinomas. Granular Cell Tumor Granular cell tumor of the breast is a rare neoplasm that clinically and mammographically may appear malignant but almost always has a benign behavior. Histology shows typically large cells with eosinophilic granular cytoplasm and an infiltrative growth pattern. Sometimes the stroma shows extensive sclerosis, and the granular cells may not be prominent: occasionally, distinction from fibrotic fat necrosis may be difficult, particularly on core biopsy. Histologic clues to the very rare granular cell tumors with a malignant behavior may be seen. A population of large transformed lymphoid cells showing infiltration through a terminal duct lobular unit. Note the preservation of preexisting structures and similarities to classic invasive lobular carcinoma. Primary breast adenocarcinoma may also metastasize to the contralateral breast or ipsilateral breast if conservation therapy has been used. Royal College of Pathologists Working Group on Breast Screen ing 1991 Pathology reporting in breast cancer screening. Office for Official Publications of the European Communities, European Commission, Luxembourg 6. Royal College of Pathologists Working Group on Breast Screen ing 1990 Breast cancer screening guidelines for pathologists. Hayward J L, Parks A G 1958 Alterations in the microanatomy of the breast as a result of changes in the hormonal environment. The majority are of Bcell phenotype, with the most common being diffuse large Bcell lymphoma, fol licle center cell lymphoma, and lymphomas of mucosa associated lymphoid tissue. Metastatic Tumors Spread to the breast from primary tumors at other sites is well recognized. It can occur with a wide range of tumors but is seen most frequently with lymphoma, melanoma, and carcino mas of the lung, stomach, and ovary. The diagnosis should be considered if the features of a tumor are unusual for a mammary primary, but a clinical history is essential in some cases that histologically may closely resemble car cinoma of the breast. The presence of in situ carcinoma and elastosis are useful features favoring mammary origin. Slavin J L, Billson V R, Ostor A G 1993 Nodular breast lesions during pregnancy and lactation. Hessler C, Schnyder P, Ozzello L 1979 Hamartoma of the breast: diagnostic observation of 16 cases. World Health Organization 1981 International histological clas sification of tumours. Burga A M, Tavassoli F A 2003 Periductal stromal tumor: a rare lesion with lowgrade sarcomatous behavior. Nielsen B B 1987 Adenosis tumour of the breast-a clinico pathological investigation of 27 cases.
Cheap malegra dxt 130mg on-line
At the dermoepidermal junction online doctor erectile dysfunction discount 130mg malegra dxt otc, note the wreath-shaped melanocyte and the "nestlets" of melanocytes in contrast with large theques that are often seen in conventional nevi. Congenital melanocytic nevi770,806-808 are common variants that present at or around birth. Characteristically, congenital melanocytic nevi tend to involve the deeper dermis as well. They tend to infiltrate or involve hair follicles, sebaceous glands, sweat ducts, follicular muscle, and the subendothelial area of blood vessels. When any of these features are present, they often are evident throughout the lesion. More cellular proliferative nodules with mitoses and even atypia, mimicking melanoma, sometimes develop in these lesions. The lesion is hyperpigmented and can be conceptualized as a crossover between epithelioid cell and blue nevus. Halo nevi814 are melanocytic lesions that present clinically with a perilesional amelanotic ring. Histologically, these lesions are infiltrated by abundant lymphocytes, which may obscure the melanocytic component. In later stage lesions, it may be difficult to identify the melanocytes that progressively disappear. Melanocyte pleomorphism may be present, and at times epithelioid cytology (Spitz-like) may be identified. Conversely, occasional clinical halo nevi may not have the characteristic inflammatory changes histologically; no special designation is offered for the histologic changes in these lesions, but this underscores the fact that a halo nevus takes its name from clinical observation and clarification should be made in pathology reports when the histologic findings do not correlate clinically. In the common variant, plump, pigmented spindle cells form fascicles among nests of ordinary nevus cells. In other variants, one or several melanocytic components that share cytologic features with either a blue nevus or a Spitz nevus are represented. Ninety-five cases, 49% of which were of the common type, were studied by Pulitzer and coworkers. Clinically, the lesions usually occur on the head and neck but may be discovered on any skin surface. Although they are widely recognized in the first two decades of life,829 they also often occur in adults. Most are solitary, but multiple lesions may occur838; these may be agminated,839-847 linear,848 or disseminated. Provided the diagnosis is correct, the lesion will not metastasize even if it is incompletely excised. Although, like other melanocytic nevi, they may be junctional, compound, or dermal, the conceptual starting point is with the compound lesions, because they are the most common and are the most easily identified. The epidermis over such lesions is rarely normal; typically some degree of acanthosis is present. Commonly, one observes thin rete ridges closely apposed to theques and dermal nests. There is a regular dermal nevus superficially, with a deeper blue nevus component. Fifteen percent were thought to have concomitant histologic features of melanocytic dysplasia, and most of these lesions were of the common type. For the common variant, the cytologic features, pattern of growth with apparent infiltration, and variable representation of the features of an atypical junctional component often mislead the pathologist. The phenotypic diversity and genetic lability of melanocytic nevus cells are manifest in combined nevi. These combined nevi should be better known because of the great potential to misdiagnose them as melanomas. This, added to some cytologic variation such as inverted melanin patterns and inflammatory responses, produces confusing histologic pictures. In recurrent or persistent nevi, pigmented nevi, if incompletely excised, often recur or persist and occasionally may cause concern to the patient and physician. In their regrowth, the melanocytes use the regenerated epidermis as a scaffold to grow along the lower layers of the epidermis. The cells appear disorganized and are usually heavily pigmented, giving the impression of a melanoma. The lesion is arranged in three zones: an intraepidermal component, a dermal scar, and the deeper layer of residual nevus. A histologic criterion useful for the diagnosis is the relative lack of cytologic atypia. The most important criterion is that the recurrent pigmentation and melanocytic proliferation is restricted to the area of the scar, respecting the boundaries of the original nevus. The pathologist should make every effort to review the original biopsy if available, because the original undisturbed melanocytic lesion should be obviously benign. Stratified melanocytic lesion with a residual dermal nevus in the deeper portions. In many theques, a crescentic space is observed adjacent to the epidermal or superficial portion of the theque, capping the melanocytes within it. Solitary melanocytes or theques may be present in the higher portions of the epidermis and may even exist within the stratum corneum; in fact, entire theques may be eliminated transepidermally. A rare subtype of pagetoid Spitz nevus has been described; however, such a diagnosis must be made with great caution. Epidermal theques often have a crescentic cleft in the superficial portion of the theque. The dermal portion may exist as nests and as single, isolated melanocytes, but rarely strands and tubules867 are identified. As the lesion is scanned from superficial to deep, nests usually predominate superficially, whereas a gradual transition to single melanocytes occurs in the deeper aspects. Variable pigmentation is present from lesion to lesion, but within a particular lesion it tends to be uniform in distribution. Stromal reactions associated with the dermal component are characteristically minimal. When stromal fibrosis does occur, it is usually uniform throughout, in contrast to the dermal components of most malignant melanomas. A mononuclear infiltrate often is interspersed within or around the lesion, and this may be pronounced to the point of being similar to a halo phenomenon,815 but it is uncommon to observe a prominent melanophagic dermatitis within or around any particular lesion, except in cases of the pigmented spindle cell variant. Rarely, clusters of melanocytes may be observed within apparent vascular spaces855 and within lymphatic spaces. Any of these findings should not be regarded, out of context, as a sign of malignancy. Whether spindle shaped or epithelioid, the melanocytes are usually monomorphous from low-power field to field, even though at high power clear differences are seen, especially among the epithelioid melanocytes. The epithelioid melanocytes have a range of morphology from those with abundant eosinophilic 1736 23 Tumors of the Skin ends of the conceptual spectrum of Spitz nevi-junctional and dermal. Junctional Spitz nevi show the same epidermal attributes observed in the compound forms. If, however, the melanocytes are entirely spindle shaped and have a pushing border, the differential diagnosis from pigmented spindle cell nevus (Reed) is impossible; in fact, for this reason, the latter is regarded as a special type of Spitz nevus. Pigmented spindle cell nevus is considered to be a special type of spindle or large epithelioid cell nevus (Spitz). Clinically, pigmented spindle cell nevi are sharply demarcated, symmetric or irregular, and dark brown to black. They are usually less than 1 cm in diameter and tend to be discovered on the limbs, especially the thigh,883 of young women in the third decade. Histologically, the lesions are junctional or compound887 and are circumscribed not only at the peripheral epidermal theques but also at the deep dermal boundary. Although theques may be eliminated transepidermally, and solitary melanocytes may be observed within the epidermis in some cases, pagetoid spread is absent. Often the melanocytes have bizarre sizes and shapes, including this myoblastoid type.
Purchase generic malegra dxt from india
A erectile dysfunction va disability compensation order malegra dxt 130mg with mastercard, this figure depicts the prototypic low-magnification appearances of this tumor type: invasive borders, tubules and cribriform structures, and sclerotic or hyaline stroma. In contrast to pleomorphic adenoma, "melting" of the basaloid cells into the stroma does not occur. The stroma is fibrous with variable amounts of myxohyaline material rather than desmoplastic, and cartilage is not formed. Sometimes extensive hyalinization results in "strangulation" of the tumor islands, to the extent that few tumor cells remain or a lace-like pattern is produced. The cribriform structures are the most characteristic feature of adenoid cystic carcinoma. They are variably-sized, smooth-contoured, discrete to coalescent islands comprising small, uniform basaloid cells punctuated by round rigid spaces, giving rise to a "Swiss cheese" appearance. The majority of the spaces are not glandular lumens but are surrounded by basaloid (basal/myoepithelial) cells. The upper field is dominated by cribriform plates, and the lower field is occupied by huge tumor islands with complex architecture. A, the cribriform island (upper and left fields) shows deposits of abundant hyaline material, with "strangulation" of the tumor cells. This pattern differs from the hyalinization seen in pleomorphic adenoma in that the process is confined to the cellular island, which itself is sharply delineated from the fibrous stroma. B, Extensive hyalinization results in "strangulation" and withering of many cribriform tumor islands. C, the prominent myxoid change of this large cribriform island resembles the "melting" pattern seen in pleomorphic adenoma. However, in contrast to the latter, this change is confined to the large cellular island, and the island itself is well demarcated from the stroma (not shown). D, In some cases, the tumor forms anastomosing trabeculae separated by abundant hyaline stroma. Ultrastructurally, these materials represent glycosaminoglycans and duplicated basal lamina. Within these cribriform islands are occasional true narrow glands lined by low cuboidal cells with eosinophilic cytoplasm. A thin eosinophilic cuticle may be present along the luminal border, and the lumen sometimes contains eosinophilic secretion. Occasionally, the glandular structures are abortive, manifesting as aggregates of vacuolated or signet ring cells. They possess round or angulated nuclei and scanty cytoplasm with indistinct cell borders. Nuclear pleomorphism is usually mild, and mitotic figures are usually few or absent. The elongated tubules are lined by a single layer of ductal epithelial cells surrounded by a single or multiple layers of basaloid cells. This is the architectural pattern in which glandular lumens are most easily and consistently found. The tubules can be apparently coiled on themselves, producing a necklace appearance. They are often embedded in abundant hyaline stroma, to the extent that they may become "strangulated. The solid pattern is characterized by smooth-contoured or focally jagged sheets and islands of closely packed basaloid cells. The basaloid cells, in comparison with those seen in the cribriform and tubular patterns, usually exhibit more significant nuclear pleomorphism and mitotic activity. A, Cribriform islands admixed with occasional tubules-this field alone is indistinguishable from pleomorphic adenoma or basal cell adenoma. Practically all the spaces in the cribriform islands (containing lightly basophilic mucin) are pseudocysts surrounded by basaloid (modified myoepithelial) cells, and they are filled with lightly basophilic mucin. B, Uncommonly, the true glandular lumens in the cribriform islands are very prominent. In this case, the glandular spaces are empty, whereas the pseudocysts contain basophilic mucin. The dedifferentiated component is usually represented by poorly differentiated adenocarcinoma, sarcomatoid carcinoma, or undifferentiated carcinoma. These cells express cytokeratin, S100 protein (usually patchy staining), actin (variably), calponin, and p63. Although c-kit is expressed in ductal cells,327,328 clinical trials using specific tyrosine kinase receptor inhibitor (imatinib) show no beneficial effects. In this example, the cystic spaces in the cribriform islands are filled with eosinophilic hyaline material rather than mucin. A, Tubules within the basaloid cell islands are abortive, being represented by groups of vacuolated "signet ring" cells. B, Basaloid cells appear separated because of accumulation of intercellular fluid or mucin. The solid pattern also predicts for distant metastasis even for early stage (T1 or T2, N0) tumor. The tubules have an inner layer of cells with eosinophilic cytoplasm and an outer layer of basaloid cells. This field alone is indistinguishable from basal cell adenoma or pleomorphic adenoma. B, the basaloid cells in the solid variant exhibit a greater degree of nuclear atypia and mitotic activity compared with conventional adenoid cystic carcinoma. Distinction from epithelial-myoepithelial carcinoma and polymorphous low-grade adenocarcinoma is discussed under their corresponding sections. The claimed value of c-kit immunoreactivity for diagnosis of adenoid cystic carcinoma has not been substantiated. The right field depicts the preexisting adenoid cystic carcinoma, comprising basaloid cells with interspersed pseudocystic spaces. The left field shows the poorly differentiated (dedifferentiated) solid tumor composed of much larger, pleomorphic, and mitotically active cells. The overall survival probabilities are 90% at 5 years, 83% at 10 years, and 67% at 20 years. Therefore lifelong follow-up is imperative even after apparently complete excision. Macroscopic Appearances Acinic cell carcinoma is often circumscribed with an incomplete capsule, but it can be multinodular or infiltrative. Microscopic Appearances Acinic cell carcinoma typically forms a solitary mass or multiple nodules and invades in broad fronts. The tumor is compactly cellular with little sclerotic stroma except for occasional traversing fibrous bands. The tumor often reveals a mixture of growth patterns comprising several cell types that recapitulate the acinarintercalated duct unit. The tumor cells are most commonly arranged in organoid sheets traversed by ramifying delicate blood vessels, sheets punctuated by microcystic spaces, cords, intertwining solid or near-solid tubules, and coalescent acini. The microcystic pattern is the most characteristic growth pattern, although it is not invariably present. Formation of these microcysts is thought to result from lack of ducts to conduct away secretions and breakdown products, causing accumulation of fluid between the cells. The microcystic spaces differ from microglandular spaces in that the surrounding cells generally lack orientation around the spaces. Similar to their normal counterparts, the neoplastic acinar cells possess basophilic granular cytoplasm and basally located nuclei. Ann Otol Rhinol Laryngol 99: 1007-1009 sion of the surrounding parenchyma or nerves and usual prominence of cribriform structures. The solid type of adenoid cystic carcinoma poses special problems in differential diagnosis from basal cell adenocarcinoma and basaloid squamous cell carcinoma (Table 7-10). Acinic Cell Carcinoma Definition Acinic cell carcinoma is a neoplasm demonstrating at least focal differentiation toward serous acinar cells. Clinical Features the most frequent sites of occurrence are the parotid gland (84%) and submandibular gland (4%), followed by the buccal mucosa, upper lip, and palate. Scattered within the basaloid islands are small to solid tubules lined by luminal cells with eosinophilic cytoplasm Rare Basaloid cells predominant Luminal cells sparse 1. Basaloid cells with pale and atypical nuclei and frequent mitoses; true glandular cells rare 2.