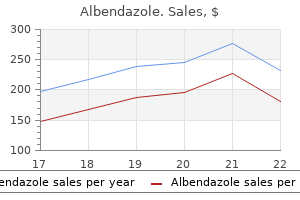
Purchase albendazole 400 mg
Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions antiviral z pack albendazole 400mg cheap. Final evaluation of tolonium chloride rinse for screening of high-risk patients with asymptomatic squamous carcinoma. Toluidine blue aids in detection of dysplasia and carcinoma in suspicious oral lesions. Comparison of chemiluminescence and toluidine blue in the diagnosis of dysplasia in leukoplakia: a cross-sectional study. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. Value of the ViziLite Plus System as a diagnostic aid in the early detection of oral cancer/premalignant epithelial lesions. Utility of chemiluminescence (ViziLite) in the detection of oral potentially malignant disorders and benign keratoses. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Clinical evaluation of chemiluminescent lighting: an adjunct for oral mucosal examinations. Analysis of surgical margins in oral cancer using in situ fluorescence spectroscopy. Advancing optical imaging for breast margin assessment: an analysis of excisional time, cautery, and patent blue dye on underlying sources of contrast. Tissue-simulating phantoms for assessing potential near-infrared fluorescence imaging applications in breast cancer surgery. Time-resolved autofluorescence spectroscopy for classifying normal and premalignant oral tissues. Imageguided surgery in head and neck cancer: current practice and future directions of optical imaging. A case series of oral precancers and cancers using white light, fluorescent light at 405 nm, and reflected light at 545 nm using the Trimira Identafi 3000. Can narrowband imaging be used to determine the surgical margin of superficial hypopharyngeal cancer Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Objective detection and delineation of oral neoplasia using autofluorescence imaging. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Understanding the biological basis of autofluorescence imaging for oral cancer detection: high-resolution fluorescence microscopy in viable tissue. Advances in fluorescence imaging techniques to detect oral cancer and its precursors. Prospective evaluation of a portable depth-sensitive optical spectroscopy device to identify oral neoplasia. Molecular imaging assisted surgery improves survival in a murine head and neck cancer model. Advances in optical adjunctive aids for visualisation and detection of oral malignant and potentially malignant lesions. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. Tracing the "at-risk" oral mucosa field with autofluorescence: steps toward clinical impact. Fluorescence spectroscopy of oral tissue: Monte Carlo modeling with site-specific tissue properties. Use of fluorescent light in detecting malignant and premalignant lesions in the oral cavity: a prospective, single-blind study. A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. Oral cancer detection using diffuse reflectance spectral ratio R540/R575 of oxygenated hemoglobin bands. Clinical evaluation of an autofluorescence diagnostic device for oral cancer detection: a prospective randomized diagnostic study. Assessing the usefulness of three adjunctive diagnostic devices for oral cancer screening: a probabilistic approach. Sensitivity of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice. The use of fluorescence technology versus visual and tactile examination in the detection of oral lesions: a pilot study. Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy. Oral mucosal disease in an Australian urban indigenous community using autofluorescence imaging and reflectance spectroscopy. Oral fluorescence imaging using 405-nm excitation, aiding the discrimination of cancers and precancers by identifying changes in collagen and elastic breakdown and neovascularization in the underlying stroma. Relevance of tumor angiogenesis patterns as a diagnostic value and prognostic indicator in oral precancer and cancer. The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions. Detecting early oral cancer: narrowband imaging system observation of the oral mucosa microvasculature. Microvascular irregularities are associated with composition of squamous epithelial lesions and correlate with subepithelial invasion of superficial-type pharyngeal squamous cell carcinoma. Narrow band imaging for detecting superficial oral squamous cell carcinoma: a report of two cases. Clinical appraisal of endoscopy with narrow-band imaging system in the evaluation and management of homogeneous oral leukoplakia. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Narrow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. A case of intraepithelial neoplasia in the oropharynx detected by endoscopic screening with narrow-band imaging videoendoscopy. Effectiveness of peroral cholangioscopy and narrow band imaging for endoscopically diagnosing the bile duct cancer. Evaluation of peroral videocholangioscopy using narrow-band imaging for diagnosis of intraductal papillary neoplasm of the bile duct. A novel approach emphasizing preoperative margin enhancement of tumor of the major duodenal papilla with narrowband imaging in comparison to indigo carmine chromoendoscopy (with videos). Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Improving visualization techniques by narrow band imaging and magnification endoscopy. Narrow-band imaging: a new tool for evaluation of head and neck squamous cell carcinomas. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. Narrow band imaging and high definition television in evaluation of oral and oropharyngeal squamous cell cancer: a prospective study. Autofluorescence improves pretreatment mucosal assessment in head and neck cancer patients. High specificity of combined narrow band imaging and autofluorescence mucosal assessment of patients with head and neck cancer. Efficacy of narrow band imaging for detection and surveillance of potentially malignant and malignant lesions in the oral cavity and oropharynx: a systematic review.
Order albendazole overnight
Similar combinatorial pharmacogenomic approaches have also been investigated in psychopharmacology and is discussed in Chapter 7 hiv infection unprotected safe 400 mg albendazole. In a meta-analysis, the G9 allele was significantly overrepresented among 317 patients with tardive dyskinesia compared to 463 patients without this adverse drug effect [299]. Furthermore, G9 allele homozygotes had higher abnormal involuntary movement scores compared to both heterozygotes and S9 allele homozygotes. This functional difference between the two alleles is reflected in stronger binding by the G allele to the endogenous opiate -endorphins, thereby affecting opioid action at the receptor site, with decreased opioid potency by a factor of two to three [302]. Decreased clinical response to opioids had been shown in carriers of the G allele. The majority of drug-target genetic associations discussed so far related to drug effectiveness. Signal Transduction Proteins Signal transduction encompasses the cascade of events following drug binding to a receptor that ultimately leads to a change in cellular response. Upon ligand binding, the receptor couples to the intracellular G-protein to elicit a cellular response. Q41L polymorphism occurs commonly in African Americans, with an allele frequency >30%. The L41 allele has been found to more effectively uncouple agonist-mediated receptor signaling and has been associated with increased transplant-free survival in African Americans with heart failure [320]. Although the 460W allele has been linked to greater blood pressure reduction with thiazide diuretics, the data are inconsistent [329,331]. The frequency of 1639A allele of rs9923231 is highest in Asians (90%) and lowest in persons of African descent (10%), with an intermittent frequency in populations of European descent (40%) [333]. The reduced enzyme activity leads to decreased dopamine degradation and subsequent increases in norepinephrine and epinephrine levels that may be associated with exaggerated levels of pain [346]. This again highlights the importance of multiple genes in mediating drug disposition and effect and the need of genotyping multiple functional polymorphisms in pharmacogenomic studies. Ion Channel Genotype One of the most often cited examples of ion channel genes with consequences for drug response are genes for the pore-forming channel proteins that affect potassium and sodium transport across the cardiac cell membrane. Moreover, there is evidence that these mutations may increase the risk for drug-induced torsades de pointes [351,352]. The 1 subunit enhances calcium sensitivity and decreases smooth muscle cell excitability, thus attenuating smooth muscle contraction. Loop diuretics are commonly prescribed for managing symptoms of fluid overload in heart failure. But, given the significant consequences of under- or overdosing loop diuretics in this disease, such information could have significant clinical value. Chapter 9 provides a more detailed discussion of these genetic variations and their effects on response to sulfonylurea agents. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. The pharmacogene variation (PharmVar) consortium: incorporation of the human cytochrome P450 (cyp) allele nomenclature database. Thus, when taking a candidate gene approach to discovery of variants impacting drug response, genes encoding proteins involved in determining drug bioavailability (transporter proteins, drug metabolizing enzymes) and response (receptor, enzyme, ion channel, and/or intracellular signaling proteins) should be considered. Genome-wide approaches to identifying determinants of drug response may reveal previously unknown proteins involved in eliciting drug response that represent potential biomarkers for predicting drug effectiveness or risk for toxicity. In addition, proteins involved in disease pathophysiology may represent attractive targets for drug development, as most often demonstrated in the area of oncology. What are examples of drug metabolism and drug-transporter genotypes that affect drug response What are examples of drugs developed based on an understanding of genes involved in disease pathophysiology. How might genes for drug metabolism, drug transport, and/or drug-target sites jointly influence drug response Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. The role of genetically determined polymorphic drug metabolism in the beta-blockade produced by propafenone. Disposition of perphenazine is related to polymorphic debrisoquin hydroxylation in human beings. Apnea in a child after oral codeine: a genetic variant - an ultra-rapid metabolizer. Codeine increases pain thresholds to copper vapor laser stimuli in extensive but not poor metabolizers of sparteine. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. Endoxifen, the active metabolite of tamoxifen, is a substrate of the efflux transporter P-glycoprotein (multidrug resistance 1). Pharmacokinetics and effect on caffeine metabolism of the proton pump inhibitors, omeprazole, lansoprazole, and pantoprazole. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Cytochrome P450 2C19 loss-offunction polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Letter by Gurbel et al regarding article, "Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement". Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. Meta-analysis: the effects of proton pump inhibitors on cardiovascular events and mortality in patients receiving clopidogrel. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Integrating interacting drugs and genetic variations to improve the predictability of warfarin maintenance dose in Chinese patients. Development of a comprehensive panel of antibodies against the major xenobiotic metabolising forms of cytochrome P450 in humans. Interindividual variability in ibuprofen pharmacokinetics is related to interaction of cytochrome P450 2C8 and 2C9 amino acid polymorphisms. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. Translational aspects of genetic factors in the prediction of drug response variability: a case study of warfarin pharmacogenomics in a multi-ethnic cohort from Asia.
| Comparative prices of Albendazole | ||
| # | Retailer | Average price |
| 1 | Gap | 721 |
| 2 | Army Air Force Exchange | 588 |
| 3 | WinCo Foods | 250 |
| 4 | Foot Locker | 134 |
| 5 | Neiman Marcus | 189 |
Albendazole 400mg cheap
There is insufficient evidence to evaluate the chemopreventive effects of vitamin E or of selenium in head and neck cancer personal hiv infection stories buy 400mg albendazole amex. Some early results on combinations of vitamin E and -carotene are encouraging (265). None of the potential intermediate biomarkers studied for the effect of chemopreventive regimes have yet been fully validated. Amongst these, micronucleated epithelial cells have received the most attention (266), and Sankaranarayanan et al. In respect to studies on retinoid chemoprevention, it may be possible to use nuclear retinoic acid receptors for this purpose (267). Attempts at chemoprevention of head and neck cancer should only be made in the context of well-planned, preferably multicenter, clinical trials. Treated patients should be monitored regularly in order to ensure that their mastication, swallowing, speaking, smiling and other functions, their physical appearance and their social integration are as good as the cancer care team can manage, and they should be screened for the possibility of new lesions. In this latter respect, References 527 Toluidine blue application may have particular utility. The cost-effectiveness of regular upper aerodigestive tract endoscopy has yet to be established. Nowhere is teamwork in cancer care more important than with treated patients, in order to maximize the quality of life for those afflicted and to ensure the best possible quality of death. Professional and community efforts to prevent morbidity and mortality from oral cancer. Helping smokers quit: a randomised controlled trial with private practice dentists. A randomised trial of smoking cessation interventions in general practice in Italy. Targeting heavy smokers in general practice: randomised controlled trial of transdermal nicotine patches. Dose effects and predictors of outcome in a randomised trial of transdermal nicotine patches in general practice. Classification of clinical autofluorescence spectra of oral leukoplakia using an artificial neural network: a pilot study. Cancer prevention in the dental practice: oral cancer screening and tobacco cessation advice. Nicotine patch use in the general population: results from the 1993 California Tobacco Survey. Effect of cessation of tobacco use on the incidence of oral mucosal lesions in a ten year follow up study of 12,212 users. Smoking tobacco, oral snuff and alcohol in the aetiology of squamous cell carcinoma of the head and neck. Mortality experience in relation to tobacco chewing and smoking habits from a 10-year follow-up study in Ernakulam District, Kerala. Tobacco habits other than smoking; betel-quid and areca-nut chewing; and some related nitrosamines. Alert for epidemic of oral cancer due to use of the betel quid substitutes gutka and pan masala: a review of agents and causative mechanisms. Intention to quit among Indian tobacco users: findings from International tobacco control policy evaluation India pilot survey. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. California Environmental Protection Agency, Office of Environmental Health Hazard Assessment. Racial differences in risk of oral and pharyngeal cancer: alcohol, tobacco and other determinants. The influence of alcohol consumption on world wide trends in mortality from upper aerodigestive tract cancers in males. Treating alcohol problems: a study of programme effectiveness and cost effectiveness according to length and delivery of treatment. Cost-effectiveness of strategies to market and train primary health care physicians in brief intervention techniques for hazardous alcohol use. Subcommittee on Health Services Research, National Advisory Council on Alcohol Abuse and Alcoholism. Improving the Delivery of Alcohol Treatment and Prevention Services: executive Summary. Bethesda: National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Department of Health and Human Services, 1997. Moderate alcohol intake, increased levels of high-density lipoprotein and its sub-fractions, and decreased risk of myocardial infarction. Alcohol use and mortality from coronary heart disease: the role of high-density lipoprotein cholesterol. Association of moderate alcohol consumption and plasma concentration of endogenous tissue-type plasminogen activator. Cancer chemopreventive effect of resveratrol, a natural product derived from grapes. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine or spirits. The positive relationship between alcohol and heart disease in Eastern Europe: potential physiological mechanisms. Oral cancer risk perception among participants in an oral cancer screening program. Dietary exposures and oral precancerous lesions in Srikakulam District, Andhra Pradesh. Food group intake and the risk of oral epithelial dysplasia in a United States population. Nutrition and Cancer Prevention; guidelines in diet,: nutrition and cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. Association between fruit and vegetable consumption and oral cancer; a meta-analysis of observational studies. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo-controlled clinical trial. Remission of oral leukoplakia and micronuclei in tobacco/betel quid chewers treated with beta-carotene and with betacarotene plus vitamin A. Chemoprevention of oral leukoplakia with vitamin A and beta carotene: an assessment. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. Evaluation of the clinical and histological effectiveness of isotretinoin in the therapy of oral leukoplakia: ten years of experience: is management still up to date and effective Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Evaluation of multiple micronutrient supplementation of oral submucous fibrosis in Karachi, Pakistan. Comparative study of efficacy of curcumin and turmeric oil as chemopreventive agents in oral submucous fibrosis; a clinical and histopathological evaluation. Comparison of curcumin with intralesional steroid injections in oral submucous fibrosis-a randomized, openlabel interventional study. Prevention of second primary tumours with isotretinoin in squamous cell carcinoma of the head and neck. A randomized double-blind placebo-controlled trial of fruit and vegetable concentrates on intermediate biomarkers in head and neck cancer. Antioxidant vitamins supplementation and mortality: a randomized trial in head and neck cancer patients. Topical oral cavity chemoprophylaxis using isotretinoin rinse: a 15-year experience. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer-a plan to move forward.
Order albendazole with amex
The update on this meta-analysis antiviral soup albendazole 400mg without a prescription, including the individual patient analyses of 17,436 patients, confirmed this finding with an absolute survival benefit of 6. This trial established the advantage of high-dose platinum (100 mg/m2) on days 1, 22 and 43 of radiation treatment. Acute and long-term toxicities have been consistently shown in this and other trials. The utility of concurrent chemoradiation was clearly established: the 10-year follow-up demonstrated that locoregional control and laryngeal preservation were superior with this approach. Patients treated with a combination of cetuximab and radiation had better progression-free survival without an increase in toxicity, one of the most feared complications seen with concurrent chemoradiation using cytotoxic agents. Prospective clinical trial data are needed because it is currently not known if locoregional control and overall survival with this regimen are better or worse than that which can be obtained with concurrent chemoradiation with cisplatin. Cetuximab has failed to provide an advantage even when added to concurrent chemoradiation using cisplatin (17). Current research is focusing on the biologic markers that predict response as well as the mechanisms of resistance to cetuximab and ways to circumvent them. Those patients who failed to respond received surgery versus chemoradiation for the initial responders. This study established that survival for induction chemotherapy followed by radiation therapy was comparable with the standard of treatment at the time-surgery followed by radiation. However, preservation of the larynx was feasible in 64% of patients who received radiation after response to induction chemotherapy. However, as discussed in the previous section, concurrent chemotherapy was found to be superior in this setting. The survival advantage of concurrent chemoradiation was statistically significant in 386 Chemotherapy patients with oropharyngeal and laryngeal tumors, with a trend of significance for oropharyngeal and oral cavity tumors. Interestingly, induction regimens appeared to be superior in the control of distant metastases. One concept under study is the use of the monoclonal antibody cetuximab instead of cisplatin. Trials to determine the optimal dose of radiation and also the potential role of surgery for these patients are also underway. Modifications of treatment protocol for these patients should only occur in the setting of a clinical trial. As with other tumor systems, the current advances in genomics, molecular medicine, immunology and tumor biology will continue to guide clinical researchers on the path toward more efficacious and less toxic therapy. Concurrent chemotherapy and radiotherapy for organ preservation in advanced larynx cancer. Chemotherapy added to locoregional treatment for head and neck squamous cell carcinoma: three meta-analyses of updated individual data. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced stage oropharynx carcinoma. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. A randomized comparison of high dose infusion methotrexate versus standard weekly therapy in head and neck squamous cancer. Cetuximab-activated natural killer cells and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. The implications of surgical complications include functional and cosmetic morbidity, prolonged hospitalization, increased treatment cost, delay in starting postoperative adjuvant therapy and mortality (5,6). Identification of factors that affect the incidence and severity of complications in head and neck cancer patients could be beneficial in preventing serious and life-threatening complications and reducing the burden of these events. Another important step is the development of new tools for the quantification of risk in an individual patient, allowing surgeons to effectively identify patients at higher risk for complications and develop strategies to improve recognition and early management of these adverse events (8). In general, complications in patients undergoing surgery for oral cancer can be divided into (1) local (those related to the surgical wound) and (2) systemic. However, some series have reported a higher incidence of complication when a higher degree of scrutiny was employed. This scale allows reproducible grading and results and allows comparison between institutions. These observations demonstrate the high incidence of complications after oral cancer surgery due to accurate and detailed data recording and reporting, as well as the quality of the data source. The co-existence of advanced medical disease, 389 390 Complications of surgical treatment and their management Table 14. Allowed therapeutic regimens are drugs as antiemetics, antipyretics, analgetics, diuretics, electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside Requiring pharmacological treatment with drugs other than those allowed for grade I complications. The risk can be reduced somewhat if the patient gives up tobacco use preoperatively or with optimization of pulmonary function by intensive respiratory therapy, rigorous postoperative pulmonary toilet and early ambulation (13,20). Cardiac complications occur in about 10% of patients with complications, usually as cardiac arrhythmias or congestive heart failure (8). Myocardial infarction occurs in less than 1% of patients undergoing head and neck surgery and is mainly related to pre-existing cardiac disease (12,21). Postoperative delirium is directly associated with poor surgical outcomes including increased length of stay and even mortality (22). Resection of the carotid artery should be performed in highly selected patients with respect to both patient and tumor parameters. However, the incidence seems to be significantly Local (surgical) complications / Intraoperative surgical complications 391 higher for patients undergoing major head and neck surgery compared to those undergoing general otolaryngology procedures (27). This is likely associated with older age and the presence of malignancy in the head and neck surgery group. Thus, a risk-based approach should be implemented in order to maximize the effectiveness of prophylaxis. The use of the Caprini risk score is recommended in selecting prophylactic measures (29). Identification of risk factors offers an opportunity for prevention of complications. Early identification of symptoms and signs and prompt institution of treatment are the pillars of effective management of these adverse events. Thus, the risk and morbidity of systemic complications can be satisfactorily controlled with collaborative multidisciplinary management to optimize care delivery before, during and after head and neck cancer surgery. When a tracheostomy tube has been placed in an emergency situation or if the procedure was complicated, a post-procedure chest radiograph should be performed to rule out the presence of pneumothorax (36,37). This risk can be minimized by limiting intravenous fluid to a volume sufficient to maintain an adequate urine output. Less common complications include intraoperative bradycardia and hypotension, which can result from manipulation of the carotid bifurcation during neck surgery. This can be counteracted by the injection of lidocaine into the adventitia in the region of the carotid bifurcation.
Discount albendazole 400mg visa
Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities hiv viral infection symptoms order albendazole with amex. Ancestral proportions and their association with skin pigmentation and bone mineral density in Puerto Rican women from New York city. Of health and human services, public health service, national center for health [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] 360 12. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Exploring variation in known pharmacogenetic variants and its association with drug response in different Mexican populations. Molecular analysis of the thiopurine S-methyltransferase alleles in Bolivians and Tibetans. Allele and genotype frequencies of genes relevant to anti-epileptic drug therapy in Mexican-Mestizo healthy volunteers. Frequencies of 23 functionally significant variant alleles related with metabolism of antineoplastic drugs in the chilean population: comparison with caucasian and asian populations. Clinical relevant polymorphisms affecting clopidogrel pharmacokinetics and pharmacodynamics: [63] [64] [65] [66] [67] [68] [69] [70] [71] 362 12. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: a cross-ethnic comparative study. Quality of life and functioning of hispanic patients with major depressive disorder before and after treatment. Relationship between personality and debrisoquine hydroxylation capacity: suggestion of an endogenous neuroactive substrate or product of the cytochrome P4502D6. Impact of population diversity on the prediction of [91] [92] [93] [94] [95] [96] [97] [98] [99] [100] hispanics with chronic hepatitis C infection. Pharmacogenetics of acute azathioprine toxicity: Relationship to thiopurine methyltransferase genetic polymorphism. Development of a pharmacogenetic-guided warfarin dosing algorithm for Puerto Rican patients. Genetic structure of pharmacogenetic biomarkers in Brazil inferred from a systematic review and [101] [102] [103] [104] [105] [106] [107] [108] [109] [110] [111] [112] [113] [114] 364 12. Pharmacogenetic research activity in Central America and the Caribbean: a systematic review. Pharmacogenetic effect of complement factor H gene polymorphism in response to the initial intravitreal injection of Bevacizumab for wet agerelated macular degeneration. Pharmacogenetics of glucocorticoid replacement could optimize the treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Cholinergic receptor nicotinic alpha 5 subunit polymorphisms are associated with smoking cessation success in women. Influence of serotonin transporter gene polymorphisms on clozapine response in Brazilian schizophrenics. Naturalistic pharmacogenetic study of treatment resistance to typical neuroleptics in European-Brazilian schizophrenics. Pharmacogenetics of risperidone and cardiovascular risk in children and adolescents. Dopamine transporter gene and response to methylphenidate in attention-deficit/hyperactivity disorder. No significant association between response to methylphenidate and genes of the dopaminergic and serotonergic systems in a sample of Brazilian children with attention-deficit/hyperactivity disorder. Adrenergic alpha2A receptor gene and response to methylphenidate in attention-deficit/hyperactivity disorderpredominantly inattentive type. Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/ hyperactivity disorder. CatecholO-methyltransferase valine158methionine polymorphism moderates methylphenidate effects on oppositional symptoms in boys with attention-deficit/hyperactivity disorder. Pharmacogenetic study of apolipoprotein E, cholesteryl ester transfer protein and hepatic lipase genes and simvastatin therapy in Brazilian subjects. N342S polymorphism is associated with response to lipid-lowering therapy in Brazilian patients with familial hypercholesterolemia. Pharmacogenetics of calcineurin inhibitors in Brazilian renal transplant patients. The role of cigarette smoking and liver enzymes polymorphisms in anti-tuberculosis drug-induced hepatotoxicity in Brazilian patients. Pharmacogenetics in Latin American populations: regulatory aspects, application to herbal medicine, cardiovascular and psychiatric disorders. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Pharmacogenetic association study of warfarin safety endpoints in Caribbean Hispanics. Frequencies of functional polymorphisms in three pharmacokinetic genes of clinical interest within the admixed Puerto rican population. Pharmacogenetic association study on Clopidogrel response in Puerto Rican hispanics with cardiovascular disease: a novel characterization of a Caribbean population. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Several academic health centers have invested in clinical pharmacogenetics and view its implementation as a first step toward incorporating genomics into routine and personalized healthcare; however, a number of challenges and barriers exist for widespread adoption. More important is the appropriate selection of variants to interrogate for a particular drug-response phenotype [5], also considering the ethnic group(s) that is being tested to maximize clinical validity. Consequently, the positive predictive value of many pharmacogenetic assays can be low. In this scenario, pharmacogenetic testing can be viewed analogous to other nongenetic clinical variables with imperfect prediction. Clinical utility of a test is a widely used measure of its usefulness in the clinic and resulting changes in health outcomes. However, given the multidimensional nature of this kind of measurement, there is rarely consensus as to its precise definition or on how to adequately demonstrate it, particularly with regard to personalized medicine and pharmacogenetics [14]. The common benchmark for interventional evidence in medicine is a prospective randomized controlled trial, yet these are often resource prohibitive for testing pharmacogenetic hypotheses, and may be unethical to conduct for strong associations of severe adverse effects associated with high-risk genotypes. The results of these trials will likely greatly influence the future of pharmacogenetic testing for anticoagulation control, and possibly other clinical scenarios with pharmacogenetic interactions. They characterized a large panel of commercially available cell lines for genes and variants commonly included in pharmacogenetic testing panels and proficiency testing surveys [21,22]. Unfortunately, for many actionable pharmacogenetic variants, these turnaround times are unacceptable for efficient implementation into routine clinical care. Moreover, given the demanding environments common to most anticoagulation clinics and cardiac catheterization laboratories, disruption of routine care by interfacing with an external clinical laboratory that has additional genetic testing logistics and unique laboratory information management systems can present further complexities for effective use of pharmacogenetic testing. Carriers were treated with prasugrel, and noncarriers and patients in the standard treatment group were treated with clopidogrel. For example, the regulatory landscape of point-of-care testing, particularly that involving genetic testing, can be complicated.
Syndromes
- Dry mouth
- Ebola fever
- Keep your shirt tucked into your pants.
- What other symptoms do you have?
- They are willing to perform the task
- Headache
- Liver function tests (serum alkaline phosphatase is most important)
- X-ray of the chest
- Severe pain in the throat
Buy albendazole with a visa
In many instances antiviral vitamins order albendazole paypal, genes for proteins involved in drug disposition, together with genes for proteins at the drug-target site, jointly influence drug response. Because drug responses are mostly determined by multiple, rather than single, proteins, recent trends of investigations on determinants of drug response have shifted from pharmacogenetics to pharmacogenomics. However, for simplicity, this chapter treats pharmacogenetics and pharmacogenomics as synonymous. Despite the scientific advances made, personalized medicine envisioned many years ago has in many cases yet to become a reality. Exceptions to this largely exists in oncology and more recently in cardiology, in which genotyping to determine clopidogrel effectiveness is starting to become routine at some large academic medical centers [3,4]. Examples of genotype-guided therapies are beginning to emerge in other therapeutic areas, which are discussed in detail throughout this book. However, significant challenges still exist in ethical, socioeconomic, regulatory, legislative, drug development, and educational issues that need to be addressed and resolved before personalized medicine can be practically and satisfactorily implemented in clinical practice on a broader scale. The goal of this chapter is to review the pharmacokinetic and pharmacodynamics basis of individualized therapy, and briefly discuss the challenges of implementing pharmacogenomics in clinical practice. Further indepth discussion of specific therapeutic areas and/or disease states, as well as ethical, socioeconomic, regulatory, legislative, drug development, technological, and educational issues will be the focus of subsequent chapters. Of these genes and pseudogenes, 42 are involved in the metabolism of exogenous xenobiotics and endogenous substances, such as steroids and prostaglandins, and 15 are known to be 4 1. Different alleles or functional variants of these polymorphisms for individual drug metabolizing genes are defined with a "star" (*) designation. In general, the clinical consequences of genetically altered-enzyme activity would depend on whether the pharmacological activity resides with the parent compound or the metabolite, and the relative contribution of the polymorphic isoenzyme to the overall metabolism of the drug. Their potential role in translating the expanding pharmacogenomic knowledge into dose requirements and therapeutic decisions will be discussed first. More recently, a software tool (originally named "Constellation" and subsequently renamed as "Astrolabe") capable of allowing rapid, automated phenotype assignment has been made available for academic research at no cost [14]. These drugs include the antianginal agent perhexiline (neuropathy) [21], the antiarrhythmic agent propafenone (proarrhythmic events) [22], and neuroleptic agents such as perphenazine (sedation and parkinsonism) [23,24]. Therefore, this fatal case underscores the importance of understanding how genes can affect pharmacological and therapeutic outcome associated with exposure to drug and/or active metabolite. However, despite strong evidence of a genotype effect on the pharmacokinetics of codeine and hydrocodone, the impact on dosage requirement is much less obvious. In addition, hydrocodone may not be a good alternative analgesic agent to codeine in these patient populations [13]. Although the clinical outcome was not described, this case underscores the importance of evaluating the modulating effect of drug interaction when utilizing genotyping in individualized therapy [52]. Similar modulating effects on other genes encoding different metabolizing enzymes are described in later sections. In contrast, the allele frequency of *3 is <1% in Caucasians and African Americans, even though the *2 occurs at a frequency of about 13% and approximately 18%, respectively, in these two ethnic groups. About 50% of the Chinese population possess either the *1/*2 and *1/*3 genotypes, and 24% have the *2/*2, *2/*3, or *3/*3 genotypes [55]. Similar frequencies of the heterozygous and homozygous variant genotypes are reported in persons of African descent. In addition, to showing a gene-dose effect in achieving desirable ranges of intragastric pH and H. Although the increased production of the active clopidogrel metabolite in carriers of the *17 allele has been associated with greater inhibition of platelet aggregation [85,86] and better clinical outcomes [87], there is also the potential of increased bleeding risk [88]. In addition, the increased response of the *17 allele has been suggested not as a direct effect, but rather attributed to that of the *1 allele [89]. Differences in patient selection for analysis likely account for the lack of association reported in two other recent meta-analyses, which included a significant number of low-risk patients, such as those with acute coronary syndrome managed medically or patients with atrial fibrillation [93,94]. Although the March 2010 version specifically addresses the implication for homozygotes, there is no guidance on the implication for heterozygotes. In light of the scientific and clinical evidences as well as the regulatory decision, several recent clinical studies addressing alternative antiplatelet agents have been initiated and are discussed in Chapter 6. I359L, respectively) in the coding region, accounting for lower enzyme activity by approximately 30% for *2 and 80% for *3 [96]. Other reduced-function alleles of potential importance included *5 (rs28371686), *6 (rs9332131), *8 (rs7900194), and *11 (rs28371685). The *1/*3 genotype occurs at a frequency of 4% in the Chinese and Japanese populations, with almost complete absence of the other genotypes (*2/*2, *2/*3, *1/*2, and *3/*3). The enzyme reduction associated with the *3 allele is greater than that with the *2 allele, with a 5- to 10-fold reduction in homozygous *3 carriers and two-fold reduction in heterozygous *3 carriers, when compared to homozygous *1 carriers. Interestingly, the effects of several reduced-function alleles appear to be substrate dependent. For the *2 allele, a significant effect was shown for clearances of acenocoumarol, tolbutamide, and warfarin but not for other substrates. Similarly, although the *8 allele has no effect on clearance of losartan, it decreases enzyme activity of warfarin and phenytoin, and exhibits an increased activity toward tolbutamide [103]. Most of the data document that the *2 and *3 alleles are associated with greater difficulty with warfarin induction therapy, increased time to achieve stable dosing, lower mean-dose requirement. In addition, with the difference in allele prevalence among different ancestral groups, the strength of association between the *2 and *3 alleles and genotypes is stronger in Caucasians [112,113]. Both *3 and *4 alleles are more common in Caucasians (with the *4 variant reportedly only found in Caucasians). On the other hand, *2 and a rare allele, *5 (rs72558196, frame-shift deletion) are exclusively found in Africans and Japanese, respectively [116,117]. Although this finding is interesting, other reports showed that genetic polymorphism of the hepatic uptake transporter plays a more important role in determining repaglinide pharmacokinetics [123]. Increased central nervous system side effects associated with variable systemic exposure of efavirenz could be the result of patients being carriers of the *6 or *18 alleles [155,164]. Incorporating determination of additional less-frequent alleles such as *26 and *29 could further improve the prediction of elevated plasma efavirenz concentrations [155,164,165]. The genotypes in these 12 patients included nine *6/*6 homozygotes, two *6/*26 heterozygotes, and one *1/*26 heterozygote. Central nervous system side effects were reported to be much less frequent at the lowerdosage regimens. Additional alleles associated with reduced enzyme activity include *7 (rs5031016, g. G71R substitution in exon 1, has also been associated with lower enzymatic activity [176]. Based on homology of their amino acid sequences, they are further classified into seven subfamilies. The polymorphism also affects plasma concentrations and clinical effects of protease inhibitors. Nevertheless, conflicting results have been reported regarding the functional and clinical significance of the polymorphism for different substrates including psychotropics (see Chapter 7), antiretroviral protease inhibitors, immunosuppressants, and anticancer drugs. In addition, the 1236C>T/2677G>T/3435C>T haplotype was shown to affect the inhibition of substrate transport and not the transport process per se [219]. Whether additional mutations resulting in loss of function, significant change in substrate specificity or functionality would have a bigger impact is not known, and awaits further studies for clarification [219,220]. Patients with the 1249G>A variant and receiving tenofovir were reported to have higher risk of drug-induced renal proximal tubulopathy, possibly a result of reduced renal drug excretion [224]. In 305 Chinese patients with hypercholesterolemia treated with 10 mg of rosuvastatin per day, a gene dose-dependent reduction in low-density lipoprotein cholesterol levels was observed in a carrier of the C421A variant [235]. N130D substitution, although conflicting results exist regarding associated changes in transport activity. Both *5 and *15 haplotypes contain the 521C allele and have been associated with reduced activity. The Met420 deletion variant commonly occurs in Caucasians and African Americans with a frequency of 18.
Buy albendazole 400mg low cost
The risk of viral contamination of cell culture-based biotechnology products can come from: 1 hiv viral infection cycle albendazole 400mg discount. Contamination of cell line, raw materials, or adventitious viruses introduced during manufacturing. This is not a common practice since it requires knowing the exact viruses to test. This is a dedicated size exclusion chromatography step that aims for several log reductions (>5) of viral count (both enveloped and non-enveloped viruses) by filtration under pressure. This size-based separation seeks to filter out larger viral particles compared to smaller, soluble biomolecules. Protein with sufficient surface hydrophilicity gets adsorbed on the resin via interaction with the immobilized ligand. Salts increase protein hydrophobicity by neutralizing and shielding the ionizable surface functional groups. The relative propensity of salts for increasing the hydrophobicity and tendency for the salting out effect is indicated by the Hofmeister series, also known as the lyotropic series. The Hofmeister series is a rank ordering of the ability of ions to precipitate or crash out proteins from their solutions. However, the increased complexity of the protein-ligand interaction may pose additional challenges for method development of the desorption phases. The ligands have a blend of aromatic or non-aromatic groups coupled with the more traditional carboxylic acid- or amine-based ion exchange properties. Hydrogen acceptor groups close to the carboxylic or amine moiety also play a role in binding to the target molecule. Several papers have also shown that desorption of the target protein from a mixed-mode ligand can be induced with electrostatic charge repulsion while accompanied by a shift in pH (Gao et al. Traditional pH or salt buffer gradients were enhanced to elute earlier by addition of urea or L-arginine to disrupt hydrogen bonding and hydrophobic moieties between the resin and the mAb. Buffer exchange happens by introduction of the target buffer into the protein solution. The semi-permeable membrane causes retention of the protein molecules in the circulation path (retentate), while certain amount of the buffer mixture is allowed to pass through the membrane (permeate). For buffer exchange, the retentate volume is held constant through pressure control mechanisms. Thus, over time, as more of the target buffer is introduced, the overall percentage of the target buffer increases and the levels of the initial buffer species remaining become minimal. To increase protein concentration, more permeate is pushed through the membrane than fresh buffer is introduced, so that the overall retentate volume is reduced and the concentration is increased. For example, the use of a diaphragm pump results in lower process shear compared to the peristaltic pump. Increase in protein concentration is typically accompanied by logarithmic increase in solution viscosity and the rate of protein aggregation, which are also functions of temperature. Proteins and peptides for parenteral administration are typically formulated as ready-to-use aqueous solutions or as a lyophilized solid mass that is reconstituted with water, isotonic dextrose solution, or isotonic sodium chloride solution immediately before administration; while proteins and peptides for inhalation and nasal routes of administration are typically formulated as dry powders (Mahato and Narang 2018). For stable molecules, nebulization of aqueous solutions is also a viable strategy. For example, Pulmozyme (dornase alpha) is a solution which is aerosolized by a nebulizer during administration. If the stability of the molecule allows, ready-to-use products are preferred from both a manufacturing standpoint and clinical administration standpoint. Formulation development is critical to enhance the stability of the molecule in solution. The development of a suitable pharmaceutical protein formulation usually involves the screening of a number of physiologically acceptable buffers, salts, chelators, anti-oxidants, surfactants, co-solvents, and preservatives (Table 8. Selection of an appropriate buffer type and strength is carried out to minimize specific/general-acid/base degradation of the protein. These components can hydrogen bond with the protein surface, stabilizing the native protein conformation. Visual inspection of filled vials or syringes for the presence of any particulate matter or critical defects in the primary components. However, modern-day manufacturing practices utilize single-use plastic liners in process tanks to avoid the risk of metal leaching and also to eliminate cleaning verification needs and crosscontamination risk. For example, development of prefilled syringes of drug product solutions requires assessment of stability with the primary packaging components, such as small quantities of silicone oil present in syringes as a lubricant-which can promote protein aggregation and instability. Additionally, the secondary packaging must be able to prevent ambient light exposure that could promote protein oxidation and potential loss of activity. This could be a problem for high dose drugs, as increases in protein concentration are often accompanied by significant increases in viscosity and physical instability. With increased viscosity, the force required to expel the drug solution from the syringe increases-reducing the syringeability (fill a syringe from the vial) and injectability (inject from the syringe into the patient) of a solution. The use of slow injection pumps in clinical trials so that the rate of injection is matched to the rate of fluid absorption from the injection site-thus enabling larger injection volumes. The use of autoinjector or semi-manual devices that enable application of high viscosity solutions with minimal force needed from the person administering the dose 3. This enzyme catalyzes the degradation of hyaluronic acid, a constituent of the extracellular matrix, increasing tissue permeability. The use of excipients, such as specific amino acids, to reduce the viscosity of high concentration biologics. The case study below exemplifies the importance of buffer exchange path on the physical stability of a high concentration protein solution. In some special cases, however, specific characteristics of the protein and sensitivity to particular buffer conditions (combination of pH and salt concentration) combined with requirements of the finished drug product. In this case study, a continuous buffer exchange process at high protein concentration destabilized an Fc fusion protein that is stable at low salt acidic pH condition and at high salt basic pH condition, but not the intermediate conditions of pH and salt concentration. This suggested subtle conformational perturbation, greater hydrophobic surface exposure, and/or propensity for conformational changes in response to other stressors such as heat. The process buffer (from the last step of downstream purification) and the formulation buffer are the two key players-the starting and the end point-of the buffer exchange process. These buffer systems are usually different due to certain practical constraints, such as the need for salt gradient purification from ion exchange columns. To maximize protein stability during manufacture and ensure long-term stability, conditions which lead to the formation of partially unfolded intermediates are avoided, and consequently, conditions which stabilize the folded state are sought. Hence, the process buffer and the formulation buffer are selected to maximize protein stability. Unfavorable processing conditions can lead to the formation of partially unfolded intermediates. Retention of these intermediates in the final filtered drug product formulation can lead to differences in protein stability. This case study shows that the shearing of the protein can lead to almost indistinguishable immediate changes, but lasting changes that impact protein stability. These findings were further confirmed by applying shear stress to the protein in the formulation buffer at different rate and/or duration and quantifying protein aggregation. Taken together, these data indicated that the shearing of the protein can lead to subtle, yet lasting, differences in the protein conformation that impact protein stability over time. It is important to emphasize that this case study highlights a special case of protein instability which is not commonly observed. In addition, this case study highlights the dependence of protein conformation and stability on processing and handling conditions during production. In such cases, freeze-drying or lyophilization is often employed to minimize the kinetics of degradation processes that occur in solutions. Many variables impact the 3 this section reproduced with permission from Mahato and Narang (2018). For example, high concentration of reacting species in the protein microenvironment can be detrimental. Further, careful optimization of residual water and protein-binding sugar concentration is required to ensure cake integrity and rapid reconstitution. In lyophilized formulations, the role of residual moisture in protein stability can be complex.
Albendazole 400 mg without prescription
Deposition and disposition of [11C]zanamivir following administration as an intranasal spray hiv infection greece discount albendazole 400mg without prescription. In vivo pharmacokinetics and pharmacodynamics in drug development using positron-emission tomography. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. Nasal mucociliary transport: New evidence for a key role of ciliary beat frequency. Pharmacoscintigraphic evaluation of lung deposition of inhaled zanamivir in healthy volunteers. Gamma scintigraphic evaluation of a novel budesonide dry powder inhaler using a validated radiolabeling technique. Effects of postural drainage, exercise, and cough on mucus clearance in chronic bronchitis. Direct labeling of ipratropium bromide aerosol and its deposition pattern in normal subjects and patients with chronic bronchitis. Efficiency of aerosol medication delivery from a metered-dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pressurized aerosol versus jet aerosol delivery to mechanically ventilated patients. The use of radiolabeled aerosols for research purposes in paediatric patients: Ethical and practical aspects. Technetium 99m radiolabeling of aerosolized drug particles from metered dose inhalers. Pulmonary deposition of salbutamol aerosol delivered by metered-dose inhaler, jet nebulizer, and ultrasonic nebulizer in mechanically ventilated rabbits. New method for easy labelling of (3-agonists in metered-dose inhalers with technetium 99m. The preparation of a radiolabeled aerosol of nedocromil sodium for administration by metered-dose inhaler that accurately preserves particle size distribution of the drug. Practical Aspects of Imaging Techniques Employed to Study Aerosol Deposition and Clearance 129 86. Deposition and clinical efficacy of terbutaline sulphate from Turbuhaler, a new multidose powder inhaler. Effect of inlet throat on the correlation between measured fine particle dose and lung deposition. Homogeneity of bronchopulmonary distribution of 99mTc aerosol in normal subjects and in cystic fibrosis patients. Comparative measurement of lung deposition of inhaled fine particles in normal subjects and patients with obstructive airway disease. Aerosol deposition in the human lung following administration from a microprocessor-controlled pressurized metered dose inhaler. Deposition and clearance: Unique problems in the proximal airways and oral cavity in the young and elderly. An improved mouthpiece to prevent environmental contamination during radioaerosol inhalation procedures. A method for measuring the absolute sensitivity of positron-emission tomographic scanners. Quantification of technetium-99m lung radioactivity from planar images [published erratum appears in Eur J Nucl Med 1993; 20(4):367]. Response of patients to fenoterol inhalation: A method for quantifying the airway bronchodilator dose. Evaluation of quantitative aerosol techniques for use in bronchoprovocation studies. Calculating concentration of inhaled radiolabeled particles from external gamma counting: External counting efficiency and attenuation coefficient of thorax. Quantitative measurements of aerosol deposition: Evaluation of different techniques. Defining lung regions for the purpose of calculating deposition to the small airways. Defining and quantitating peripheral lung deposition using radiolabeled tracers and 2D imaging. Comparisons of planar and tomographic gamma scintigraphy to measure the penetration index of inhaled aerosols. Three-dimensional description of pulmonary deposition of inhaled aerosol using data from multimodality imaging. Can lung deposition data act as a surrogate for the clinical response to inhaled asthma drugs Lung deposition of 18-F-fluticasone propionate in normal subjects using positron emission tomography. The preferential deposition of inhaled isoproterenol and propranolol in asthmatic patients. Standardization of inhalation provocation tests: Two techniques of aerosol generation and inhalation compared. The bronchial response, but not the pulmonary response, to inhaled methacholine is dependent on the aerosol deposition pattern. The relationships of aerosol deposition, lung size, and the rate of mucociliary clearance. Consensus statement from a workshop of the British Association for Lung Research, held at the Institute of Biology, London, on April 17, 1998. Description of pulmonary deposition of radiolabeled aerosol by airway generation using a conceptual three-dimensional model of lung morphology. Thus, inhalation therapy intends to obtain desired effects at smaller doses than those necessary after systemic administration, thereby reducing systemic side effects. This article will discuss the relationships between pulmonary selectivity, pharmacokinetic and pharmacodynamic drug properties, including device and formulation related factors. In addition, various pharmacokinetic and pharmacodynamic approaches suitable to characterize the fate of inhalation drugs will be reviewed. These include non-compartmental, compartmental, and physiologically based pharmacokinetic approaches. Drug deposited in the oropharynx will be swallowed and becomes subject to oral absorption. The human lung can be divided into at least two major physiological zones differing in airway diameter and cellular composition (Patton 1996; Tronde et al. According to these zones, lung deposition of drug is often classified as either central or peripheral. Depending on the physicochemical properties of the inhaled dose, drug particles will deposit in central and peripheral portions of the lung with smaller particles depositing more peripherally. Deposited drug will subsequently dissolve and interact with relevant receptors to stimulate the desired drug effects. After deposition in the central or peripheral lung, drug particles can dissolve in the epithelial lining fluid where the dissolution rate can be described by an empirical first-order dissolution constant or by the Nernst-Brunner equation (with solubility and particle surface area as input parameters). Free dissolved drug molecules are absorbed into the lung tissue via first-order absorption (affected by permeability and surface area). After absorption into the lung tissue the unbound drug fraction can induce pulmonary response or distribute into the vasculature from where it becomes systemically available by the cardiac output (Qca) in the peripheral lung or the bronchial blood flow (Qbr) in the central lung. This model is therefore not solely centerd around plasma concentrations (Sykes and Charlton 2012), as generally the case for models evaluating the effects of systemically acting drugs; instead, the proposed model links pulmonary effects to free concentrations in the lung while systemic side effects are related to plasma concentrations. Pulmonary targeting was accordingly defined as the difference between cumulative receptor occupancy of receptors in the lung and in the systemic circulation.
Buy albendazole without a prescription
On one hand how soon after hiv infection symptoms buy 400 mg albendazole, these examples definitely underscore the importance of understanding the multi-genic nature of drug efficacy. However, the everexpanding list of variants not only adds to complex interpretation that can be challenging and frustrating to most practitioners, but also could be perceived by payers that the existing variants already included in test panels are not as important as they purport to be. Therefore, the unintended consequence of more data could be generation of scientific uncertainty for experts deciding on clinical guidelines for potential adoption of specific biomarkers in practice, and opportunity for payers to decline reimbursement for specific biomarkers, because they need to evaluate more clinical utility and cost-effectiveness studies that incorporate even more patients to account for the additional variant(s). Evaluation of Cost-Effectiveness For many healthcare facilities and systems, demonstration of cost-effectiveness of any test or procedure is critical prior to its implementation. Ideally, the pharmacogenomic biomarker not only will result in cost-effective improved clinical care in patients who will benefit from individualized therapy with the drug, but also will lead to avoidance of costineffective treatment for patients who likely will not benefit from the drug, either because of lack of response or increased adverse drug reactions. Given the differences in revenue generation between a pharmacogenomic diagnostic companion test and a drug, conceivably, there could be much less incentive for pharmaceutical companies to include thorough cost-effectiveness analysis as part of drug development. However, demonstration of cost effectiveness of pharmacogenomics-based therapy can take different approaches. These approaches include comparing per-patient cost for specific clinical outcome between genotype-based regimen and standard regimen, as shown by the study of Furata et al. Examples of how claims and clinical data can be used to establish direct and indirect cost benefits are reviewed in chapter 14. The economic impact and cost-effectiveness of screening can be affected by different variables. Finally, in addition, to using multiple approaches and/or models, it is also important to consider variables such as prevalence of a specific variant in a population (which impacts cost-effectiveness) and cost of alternative treatment approaches when evaluating cost-effectiveness. This classification comprises of three categories: (1) test required before the drug is prescribed, either for predicting efficacy or toxicity; (2) test recommended; and (3) test available only for information purposes. Unfortunately, the "for information only" classification is confusing to most practitioners. As such, expert committees do not endorse pharmacogenomics testing for drugs in the third category (primarily based on lack of sufficient supporting evidence and the regulatory classification of no mandatory testing), and such nonendorsement is extensively used by payers as the basis of non-reimbursement for genotyping. The lack of clarity, in particular, had led to the conclusion by many payers that despite the scientific evidence, pharmacogenomics testing is "experimental" and, therefore, payers should not be responsible for experimental medical procedures. In addition, most reporting from these panels did not provide sufficient information on variants, resulting in a distinct possibility that two panels measuring the same allele may report conflicting phenotypic prediction of metabolic status [35]. Within the United States, tests for a pharmacogenomic biomarker are performed either as a test developed by a clinical laboratory, or as an in vitro diagnostic device, each with its own regulatory oversight. Although currently there is no formal regulatory process for submission of companion diagnostic tests, the well-established medical test and device regulatory process within the Office of In Vitro Diagnostic Devices seems amenable for application to approval of biomarkers. Integration of Testing Within the Healthcare Environment There are two practical aspects that need to be addressed before pharmacogenomic markers can be successfully utilized in any healthcare setting. Although most clinicians have a positive impression with the potential implication of pharmacogenomics testing for their practice [37,38], integration of the testing logistics and procedure within the existing workflow of specific healthcare practice poses a logistical challenge. Low volume of the diagnostic test may not justify inhouse testing in institutional 112 4. The ideal point-of-care performance for rapid decision-making at the bedside or within the clinic is not available at most hospitals. The inevitable outcome is longer turnaround time for test results coming from external clinical laboratories or research institutions. An alternative approach would be the adoption of preemptive genotyping [2,41], which helps to optimize workflow in the clinic environment [41,42]. The second practical consideration relates to managing and using the information in a clinically relevant manner for patient care. A recent survey of primary care physicians at the Mayo Clinic revealed an overall lack of comfort with the clinical decision support in place [46], and underscores the importance of further refining the alert system [47]. With availability of test results comes the needs of interpretation and education of clinicians. Most pharmacogenomic diagnostic tests report genotype result, the interpretation of which is usually not difficult, especially for deciding the appropriateness of a specific drug for a patient. The interpretation is more complicated and challenging when the test result is used for dosing adjustment. Nevertheless, although these algorithms can be useful, they are not without limitations. Although the algorithm approach has been successfully used in inpatients receiving multiple-drug therapy [53], most data have been primarily derived from outpatients receiving stable warfarin-dosage regimens. Not surprisingly, different, albeit not statistically significant, dosage requirements were obtained with various algorithms [54], likely reflecting the inconsistency in the choice of specific nongenetic variables among these algorithms. Nevertheless, this practical challenge of data interpretation can be mitigated with appropriate level of educational support and consultation [4,5]. Much like other clinical diagnostic tests, patients expect clinicians to be able to explain the pharmacogenomic diagnostic-test results and answer their questions. However, a survey of more than 10,000 physicians conducted in 2008 found that only 10% felt adequately trained to apply genetic information in clinical practice and only 26% had received pharmacogenomics education during their medical school or postgraduate training [56]. A more recent survey for the European pharmacogenomics implementation project "Ubiquitous Pharmacogenomics" identified similar self-identified lack of sufficient knowledge on pharmacogenomics from 40% of respondents [57]. In their accreditation guidelines update (version 2, 1/23/2011), the Accreditation Council for Pharmaceutical Education listed pharmacogenomics as part of professional curriculum course work [58]. Although most pharmacy schools have pharmacogenomics courses or materials in place, this is not the case with medical schools [59,60]. Hopefully, additional clinical practice guidelines from diverse groups of organizations and expert panels would pave the way to greater extent of implementation. The current landscape of pharmacogenomics education and guidelines is further described in chapter 15. The challenge of informing the patient is further amplified with the proliferation of available direct-to-consumer tests, especially within the psychiatry discipline. Such educational tools, upon further refinement, could help facilitate informed consent by the patient prior to discussion about the need of pharmacogenomic testing for specific drug. Further work in this area of "engaged patient care decision-making" would be much welcomed. Reimbursement Issues the successful implementation of pharmacogenomic biomarkers in clinical practice not only involves multidisciplinary coordination from physicians, pharmacists, and clinical laboratories, but also requires efforts from the payer. With the high cost of providing healthcare, the reimbursability of any particular test plays a significant role in deciding its implementation status in clinical practice. For most pharmacogenomic biomarkers, the ideal analysis might not be available until years after the diagnostic test is marketed. With this designation, different studies utilizing real-world claims data from patients have been conducted with several commercially available pharmacogenomic tests, including those used for therapy with warfarin and psychotropic agents, to assess the clinical and economic impact of pharmacogenomic testing. Despite the consistency in relying on existing conclusive evidence that links pharmacogenomic testing with health outcomes, there is significant variation among payers in reimbursement policy and the extent of coverage. Most payers consider conclusive evidence of link between the diagnostic test and health outcome to be much more important than evidence of test accuracy in identifying subpopulations of interest. Interestingly, the authors reported that most payers indicate test cost, medication adherence, and off-label use are not factors in their consideration for reimbursement. It is clear that, currently, payers are reluctant to pay for the diagnostic tests (most costing $500), even though they will pay for the more expensive drugs. Such a stance would pose much less incentive for diagnostic companies to develop biomarkers, as they usually have less financial resources than pharmaceutical companies. Likewise, a similar paradigm shift might be applicable for reimbursement of companion diagnostics. Instead of reimbursing the same rate for every patient testing for a pharmacogenomic biomarker, it might be less of a financial burden for payer to institute a differential reimbursement based on indication. This could provide additional incentive to use pharmacogenomic biomarkers as the equivalent of a differential diagnostic test to identify patients who will benefit most from genomics-guided personalized drug therapy. The financial cost of the one-time test should be easily covered through cost savings associated with not using the drug when it is ineffective or harmful in specific patient populations. As a result, concerns have been raised regarding individual right for privacy, as well as potential for discrimination and ineligibility for 116 4.
Order discount albendazole on line
Advances in statistical methodology and computer technology have converged so that we now have the ability to use tools such as nomograms to not only predict outcomes of interest in individual patients antiviral film buy cheap albendazole 400 mg on-line, but also compare groups of patients. Staging of head and neck cancers: is it time to change the balance between the ideal and the practical Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. A comparison of published head and neck stage groupings in carcinomas of the oral cavity. The effects of age on survival and other parameters in squamous cell carcinoma of the oral cavity, pharynx and larynx. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States). Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: a multicentric European study. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Cervical lymph node metastases-diagnostic, therapeutic, and prognostic implications. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Molecular markers in laryngeal squamous cell carcinoma: towards an integrated clinicobiological approach. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Morphological and wavelet features towards sonographic thyroid nodules evaluation. Computer-aided diagnosis for the differentiation of malignant from benign thyroid nodules on ultrasonography. The use of artificial intelligence to identify people at risk of oral cancer and precancer. An artificial neural network improves prediction of observed survival in patients with laryngeal squamous carcinoma. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Nomograms are superior to staging and risk grouping systems for identifying highrisk patients: preoperative application in prostate cancer. Nomogram for deciding adjuvant treatment after surgery for oral cavity squamous cell carcinoma. Nomogram for predicting malignancy in thyroid nodules using clinical, biochemical, ultrasonographic, and cytologic features. Nomogram to aid selection of patients for short-stay thyroidectomy based on risk of postoperative hypocalcemia. Pretreatment probability model for predicting outcome after intraarterial chemoradiation for advanced head and neck carcinoma. Development and validation of a nomogram for prediction of survival and local control in laryngeal carcinoma patients treated with radiotherapy alone: a cohort study based on 994 patients. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Assessing the performance of prediction models: a framework for traditional and novel measures. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. Everything you always wanted to know about evaluating prediction models (but were too afraid to ask). The goal of the multidisciplinary team is to maximize tumor control and minimize treatment-related sequelae, which impact upon the quality of life. Nowhere in the head and neck region is this more important than in the oral cavity and the oropharynx. The role of the dental surgeon and the prosthodontist is crucial from the day of initial diagnosis until after completion of treatment, rehabilitation and, thereafter, lifelong for management of the long-term sequela and complications of treatment. Intraoperative and perioperative dental management is crucial to the successful outcome of a surgical procedure. Similarly, preradiation assessment of dentition, fabrication of protective devices during radiation and post-radiation management of the radiated dentition are crucial to the successful outcome of treatment with radiation with none or minimal impact of radiation exposure on the teeth. Management of the dentition in patients receiving chemotherapy is equally important and complex. Avoidance of complications due to long-term bisphosphonate therapy is an integral part of the overall care of the patient. These personalized, individually fabricated devices significantly improve form and function and thus the quality of life after treatment of patients with head and neck cancers (1). This should be done prior to any invasive intervention, during the treatment planning phase, either preoperatively or preradiation. The initial examination by the dentist involves assessment of the teeth clinically and by at least a screening orthopantomogram. Septic teeth that are not salvageable are extracted, unless they are in a tumor-bearing area. If possible, restorative dentistry should be completed prior to radiation treatment. Ideally, a period of at least 2 weeks should be available to allow healing of soft tissue manipulations and dental extractions so that mucosal integrity is restored before treatment begins. It must be emphasized that dental extraction in the proximity of the tumor opens up a route for implantation of malignant cells through the alveolar process/dental socket and therefore it should be avoided. However, prolonged dental treatments should be delayed until after completion of cancer therapy because it is imperative that the cancer treatment takes priority over long-term elective dental treatment. The general practice dentist and hygienist can use the interval between initial screening appointment and commencement of external beam radiation therapy to complete all hygiene procedures such as scaling, polishing, soaking, root planning and curettage. Overhanging and faulty restoration can be removed and replaced appropriately and ill-fitting dentures should be corrected. Home care should include infective daily plaque removal and use of soft toothbrushes with application of high-potency fluoride. Fluoride trays are made for most patients and the need for daily topical fluoride is emphasized to reduce the risk of dental caries. Patients are instructed to floss daily and brush their teeth after every meal, which includes liquid supplements, because they contain cariogenic carbohydrates. A toothpaste or gel that contains neutral 1% sodium fluoride is preferred over stenosed fluoride, which has an unpleasant taste and adverse effects such as sensitivity of the teeth and gingiva. One may expectorate as much as desired, but patients should not rinse the mouth clean in the evening, leaving a thin film of fluoride on the teeth while sleeping (1). Dental extractions should be done as soon as possible in those patients that require them (Table 22. Teeth may be extracted if they are decayed and unrestorable, lack opposing teeth, prosthetically useless and are likely to be lost during the course of treatment or in patients who are unwilling to carry out the rigorous oral care and hygiene regimen required. In addition, partly erupted third molar teeth and those with severe periodontal disease should be removed to prevent pericoronitis or other infections. Teeth with periodontal inflammatory disease or non-vital teeth may be kept if the patient is willing to undergo root canal treatment. As previously mentioned, all extractions should be completed in time for mucosal healing to occur prior to starting cancer treatment. These should be smoothed out by filing and, if possible, the mucosa of the gingiva at the extraction site should be closed with sutures (2).