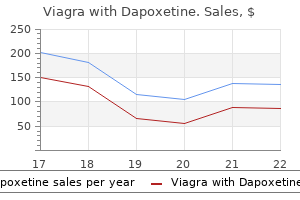
Purchase viagra with dapoxetine 100/60mg
Thus impotence beavis and butthead purchase viagra with dapoxetine 50/30 mg online, the central lateral nucleus projects mainly to parietal and temporal association areas, the paracentral nucleus to the occipitotemporal and prefrontal cortex and the central medial nucleus to the orbitofrontal and prefrontal cortex and to the cortex on the medial surface. Many cells throughout the anterior nuclei have branched axons, which pass to both the cortex and the striatum. The posterior intralaminar nuclei receive a major input from the internal segment of the globus pallidus. Additional afferents come from the pars reticulata of the substantia nigra, the deep cerebellar nuclei, the pedunculopontine nucleus of the midbrain and possibly the spinothalamic tract. The central lateral nucleus receives afferents from the spinothalamic tract, and all component nuclei receive fibres from the brain stem reticular formation, the superior colliculus and several pretectal nuclei. Afferents to all intralaminar nuclei from the brain stem reticular formation include a prominent cholinergic pathway. They appear to mediate cortical activation from the brain stem reticular formation and play a part in sensorimotor integration. Damage to the intralaminar nuclei may contribute to thalamic neglect-that is, the unilateral neglect of stimuli originating from the contralateral body or extrapersonal space. The latter has been targeted in humans for the neurosurgical control of pain and epilepsy. Bilateral injury to the posterior intralaminar nuclei leads to akinetic mutism, with apathy and loss of motivation. A second syndrome associated with damage involving the intralaminar nuclei is that of unilateral motor neglect, in which there is contralateral paucity of spontaneous movement and motor activity. Cells within these regions respond to visual, somatic or auditory stimuli with a latency, suggesting that these properties arise from activation by thalamocortical axon collaterals. The projections into the main thalamic nuclei broadly, but not entirely, reciprocate the thalamoreticular connections. The reticular nucleus is believed to function in gating information relayed through the thalamus. In this chapter, the midline group of nuclei includes those medial thalamic structures ventral to the central medial nucleus-that is, the rhomboid and reuniens nuclei, together with the parataenial nuclei more dorsolaterally. The midline nuclei receive subcortical afferent fibres from the hypothalamus, the periaqueductal grey matter of the midbrain, the spinothalamic tract and the medullary and pontine reticular formations. They are the major thalamic target of ascending noradrenergic and serotoninergic axons from the locus coeruleus and raphe nuclei, respectively, and they also receive a cholinergic input from the midbrain. Efferents from the midline nuclei pass to the hippocampal formation, the amygdala and the nucleus accumbens. Additional thalamocortical axons reach the cingulate and possibly the orbitofrontal cortex. The dual cortical and basal nuclear relationship of these nuclei has often led to their being considered part of the intralaminar system. The relationships of the midline nuclei clearly identify them as part of the limbic system. There is some evidence that they may play a role in memory and arousal and, pathologically, may be important in the regulation of seizure activity. The reticular nucleus is a curved lamella of large, deeply staining fusiform cells that wraps around the lateral margin of the thalamus, separated from it by the external medullary lamina. Anteriorly, it curves around the rostral pole of the thalamus to lie between it and the prethalamic nuclei, notably the bed nucleus of the stria terminalis. The nucleus is so named because it is crisscrossed by bundles of fibres that, as they pass between the thalamus and cortex, produce a reticular appearance. The nucleus is thought to receive collateral branches of corticothalamic, thalamocortical and probably thalamostriatal and pallidothalamic fibres as they traverse it. It receives an additional, probably cholinergic, afferent pathway from the nucleus cuneiformis of the midbrain. Broadly speaking, the afferents from the cortex and thalamus are topographically arranged. The Reticular Nucleus the hypothalamus consists of only 4 cubic centimetres of neural tissue, or 0. Nevertheless, it contains the integrative systems that, via the autonomic and endocrine effector systems, control fluid and electrolyte balance, food ingestion and energy balance, reproduction, thermoregulation and immune and many emotional responses. The hypothalamus extends from the lamina terminalis to a vertical plane posterior to the mammillary bodies, and from the hypothalamic sulcus to the base of the brain beneath the third ventricle. Laterally, it is bordered by the anterior part of the subthalamus, internal capsule and optic tract. Structures in the floor of the third ventricle reach the pial surface in the interpeduncular fossa. From anterior to posterior, they are the optic chiasma, tuber cinereum, tuberal eminences and infundibular stalk, mammillary bodies and posterior perforated substance. The last lies in the interval between the diverging crura cerebri, pierced by small central branches of posterior cerebral arteries. Within it is the small interpeduncular nucleus, which receives terminals of the fasciculus retroflexus of both sides and has other connections with the mesencephalic reticular formation and mammillary bodies. The mammillary bodies are smooth, hemispherical, pea-sized eminences lying side by side, anterior to the posterior perforated substance. The tuber cinereum, between the mammillary bodies and the optic chiasma, is a convex mass of grey matter. From it, the median, conical, hollow infundibulum becomes continuous ventrally with the posterior lobe of the pituitary. Around the base of the infundibulum is the median eminence, which is demarcated by a shallow tubero-infundibular sulcus. Hypothalamic lesions have long been linked with widespread and bizarre endocrine syndromes and with metabolic, visceral, motor and emotional disturbances. The hypothalamus has major interactions with the neuroendocrine system and the autonomic nervous system, integrating responses to both internal and external afferent stimuli with the complex analysis of the world provided by the cerebral cortex. The hypothalamus controls the endocrine system in a variety of ways: through magnocellular neurosecretory projections to the posterior pituitary, through parvocellular neurosecretory projections to the median eminence (these control the endocrine output of the anterior pituitary and thereby the peripheral endocrine organs) and via the autonomic nervous system. The posterior pituitary neurohormones vasopressin and oxytocin are primarily involved in the control of osmotic homeostasis and various aspects of reproductive function, respectively. The hypothalamus influences both parasympathetic and sympathetic divisions of the autonomic nervous system. In general, parasympathetic effects predominate when the anterior hypothalamus is stimulated; sympathetic effects depend more on the posterior hypothalamus. Stimulation of the anterior hypothalamus and paraventricular nucleus can cause decreased blood pressure and decreased heart rate. Stimulation in the anterior hypothalamus induces sweating and vasodilatation (and thus heat loss) via projections that pass through the medial forebrain bundle to autonomic centres in the brain stem and cord. Stimulation in the posterior part of the hypothalamus induces sympathetic arousal with vasoconstriction, piloerection, shivering and increased metabolic heat production. Circuitry mediating shivering is located in the dorsomedial posterior hypothalamus. This does not imply the existence of discrete parasympathetic and 267 Chapter 15 Section V / the Cerebrum Gyri recti of the frontal lobe Olfactory tract Optic nerve Optic chiasma Anterior perforated substance Optic tract Tuber cinereum with attached infundibular stem (pituitary stalk) Mammillary body Posterior perforated substance Oculomotor nerve Trochlear nerve Crus cerebri Pons Trigeminal nerve. Hypothalamic Nuclei the hypothalamus contains a number of neuronal groups that have been classified on phylogenetic, developmental, cytoarchitectonic, synaptic and histochemical grounds into named nuclei, many of which are not clearly delineated, especially in the adult. Although it contains a few large myelinated tracts, many of the connections are diffuse and unmyelinated, and the precise paths of many afferent, efferent, and intrinsic connections are uncertain. The hypothalamus can be divided anteroposteriorly into chiasmatic (supraoptic), tuberal (infundibulo-tuberal) and posterior (mammillary) regions and mediolaterally into periventricular, intermediate (medial) and lateral zones. Between the intermediate and lateral zones is a paramedian plane that contains the prominent myelinated fibres of the column of the fornix, the mammillothalamic tract and the fasciculus retroflexus. For this reason, some authors group the periventricular and intermediate zones as a single medial zone. In the anterior wall of the ventricle is the vascular organ of the lamina terminalis (organum vasculosum), which is continuous dorsally with the median preoptic nucleus and subfornical organ.
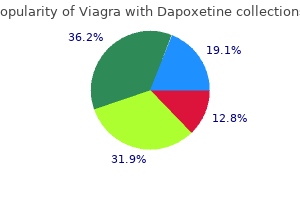
Order viagra with dapoxetine 100/60mg with amex
Early filling of the left portal vein is due to an arterioportal fistula from biopsy erectile dysfunction pills generic purchase viagra with dapoxetine without prescription. While these were unsuspected findings, this patient may have a forme fruste of tuberous sclerosis complex. Similar lesions were present in the spleen and grew rapidly with a fatal outcome within several months. The liver is dysmorphic with a normal density but hypertrophied caudate lobe and a shrunken peripheral liver of lower density. Note the dysmorphic liver and the central dot sign representing the portal vein surrounded by the dilated intrahepatic ducts. Sonography the next day confirmed innumerable focal hypoechoic metastases & no evidence of steatosis. The caudate and deep right lobe segments are hypertrophied with peripheral liver scarring. The very dense focus is residual Lipiodol that had been given as part of intraarterial chemotherapy. Note the calcification within the large, poorly enhancing scar, and cardiophrenic lymphadenopathy. Like many large hemangiomas, this one has a central, nonenhancing scar with a small focus of calcification. Grayscale transabdominal ultrasound of the liver shows typically echogenic liver metastases from colonic carcinoma. Patient has ascending cholangitis, and gas may enter the abscesses from gas-containing ducts or result from infection itself. The larger lesion has gas due to tumor infarction as a result of chemotherapy; there was no infection. One of these has gas within it as well as surgical clips, the latter the result of bleeding after ablation that required intervention. The gas bubbles are not the result of infection but rather infarction of hepatic tissue (or tumor). This is oxidized surgical gelatin (Surgicel) that was used as a hemostatic device & left in place. Within the abscess, there is a tightly packed collection of gas bubbles that represents oxidized surgical cellulose (Surgicel) that had been placed to control bleeding at surgery. These findings of colonic pneumatosis persisted for months as an asymptomatic finding. The presumed etiology was the use of steroids and other immunosuppressive medications. These findings would be suggestive of cirrhosis in a younger individual but may be normal, especially in the elderly. The liver is small, with varices, widened fissures, and rounded contours due to peripheral hepatic scarring and hypertrophy of the more central segments. The portal vein branches are surrounded by a collar of low density, some of which probably represents periportal edema. However, there are also discrete lowdensity focal lesions that represent periportal cysts within the bile duct walls. Ascites, peripheral hepatic damage, and central hypertrophy are typical findings of Budd-Chiari. On lower sections, hepatic arteries were enlarged and small cystic kidneys were noted. All findings were related to fibropolycystic disease of the liver and kidneys, including congenital hepatic fibrosis. There is no large mass evident at the site of obstruction, typical of a hilar (Klatskin) tumor. The portion of the liver in apposition to the gallbladder shows abnormal bright enhancement, indicating increased perfusion and indirectly suggesting inflammation. The metastatic foci that are blocking the portal venous tributaries are less clearly seen. The hyperperfused left lobe is presumably due to the sump effect of the abscess, rather than portal vein occlusion, because the left portal vein is patent. The anterior branch of the right portal vein is occluded and the accompanying hepatic arterial branch is enlarged. The hyperenhanced right lobe is due to increased arterial flow delivered contrast opacified blood, while the remainder of the liver is perfused more by less opacified portal vein flow. Transient hepatic intensity differences: part 1, Those associated with focal lesions. Transient hepatic intensity differences: part 2, Those not associated with focal lesions. The lesions are almost cystic in appearance, reflecting the cystic nature of the primary tumor. Indications that the hepatic lesions are not simple cysts include the subtle wall thickening and irregularity. One of the larger cysts has a thin septum, but there is no nodularity of the wall. The lesions are often not perfectly spherical, and many have visible nodular enhancement within their walls. In this febrile, Hispanic immigrant, amebic abscess was considered and confirmed by serology. Note the central dot sign representing the hepatic artery enveloped by the cystic bile duct dilations. Diffuse slight hyperintensity suggests the presence of lipid (fat) within the lesion. Sections through the abdomen (#7-10) show numerous small, hypodense spherical and wedge-shaped lesions in the liver (arrows, #7-9) and spleen (curved arrows, #7-9), along with wedge-shaped and striated hypodense lesions in the right kidney (open arrow, #10). The renal involvement is less typical and probably represents tubulointerstitial nephritis. A more common renal manifestation of sarcoid is nephrocalcinosis +/nephrolithiasis. These were encapsulated and heterogeneously hypervascular on other phases of imaging. The hypodensity of the mass is indicative of lipid content, another typical feature of hepatic adenoma. Note the hyperemia of the anterior segments of the right hepatic lobe, due in part to occlusion of the anterior branch of the right portal vein. Note the presence of numerous septa within the mass, representing scolices, or daughter cysts within the larger endocyst. Also note the central scar and a pseudocapsule, composed mostly of dilated, draining veins. The ducts lie on only one side (usually anteromedial) of the portal vein branches. In this 60-year-old man with ulcerative colitis and primary sclerosing cholangitis, portions of the left hepatic ducts are more dilated than the common duct. Recurrent pyogenic cholangitis can cause periportal edema in addition to massively dilated bile ducts. Irregular dilation of intrahepatic ducts is result of hepatic intraarterial chemotherapy. These are classic signs of hepatic artery stenosis or thrombosis following liver transplantation. This is a common finding in recent liver transplant recipients and does not imply injury to the allograft. Note collateral veins in this 58-year-old woman who had an autologous bone marrow transplantation prior to becoming hypercoagulable. Steatosis often favors the perivascular planes and "spares" the liver adjacent to the gallbladder fossa.
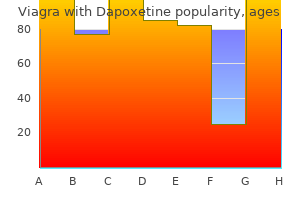
Discount viagra with dapoxetine 50/30 mg amex
Stellate cells are located in the superficial molecular layer erectile dysfunction foods 50/30mg viagra with dapoxetine with amex, and their axons synapse with the shafts of Purkinje cell dendrites. Both stellate and basket cells receive excitatory synapses from parallel fibres passing through their dendritic trees. Their somata receive synapses from Purkinje cell recurrent collaterals and from climbing and mossy fibres, as well as from the parallel fibres. Basket cell axons increase in size away from their somata and run deep in the molecular layer just above the Purkinje cells. Continuing for approximately 1 mm, each covers the territories of 10 to 12 Purkinje neurones. Branches from each basket cell axon also extend in the direction of the long axis of the folium to an additional three to six rows of Purkinje neurones, flanking the axon. It follows that as many as 72 Purkinje cell neurones may receive synapses from a single basket neurone. Most Golgi cell somata occupy the superficial zone of the granular layer, adjoining the Purkinje cell somata. Unlike Purkinje cells, the dendritic trees of Golgi cells are not flattened, appearing much the same in transverse and longitudinal foliar section. In both planes they overlap the territories of several neighbouring Purkinje and Golgi cells. Some Golgi dendrites, however, divide in the granular layer and join cerebellar glomeruli, where they receive excitatory synaptic contacts from mossy fibres. The axon of the Golgi cell arises from the base of the cell body or proximal dendrite and immediately divides into a profuse arborization that extends through the entire thickness of the granular layer. The volume of the territory occupied by the axonal ramifications corresponds approximately to that of its dendritic tree in the molecular layer and it overlaps with the axonal arborizations of adjacent Golgi cells. The main synaptic input to Golgi cell dendrites is from parallel fibres in the molecular layer. Purkinje cell recurrent collaterals and mossy and climbing fibres also terminate on their proximal dendrites and, more sparsely, on their somata. Granule cells give rise to three to five short dendrites that end in claw-like terminals within the synaptic glomeruli. The fine axons of granule cells enter the molecular layer and branch at a T-junction to form parallel fibres passing in opposite directions over a distance of several millimetres. Terminals located along the parallel fibres give them a beaded appearance and are sites of synapses on the dendrites of Purkinje, stellate, basket and Golgi cells in the molecular layer. It has been estimated that 250,000 parallel fibres cross a single Purkinje dendritic tree, although every parallel fibre may not synapse with the dendritic tree it crosses. Two very different excitatory inputs serve the cerebellar cortex: climbing fibres and mossy fibres. Olivocerebellar fibres cross the white matter and enter the granular layer, where they branch to form climbing fibres. There are about 10 times as many Purkinje cells as there are cells in the inferior olive, so each olivocerebellar fibre branches into approximately 10 climbing fibres. Individual climbing fibres pass alongside the soma of a Purkinje cell and then branch to make numerous synapses on the short, stubby spines that protrude from the proximal segments of Purkinje cell dendrites. Mossy fibres take their origin from the spinal cord, trigeminal, dorsal column and reticular nuclei of the medulla and from the pontine tegmentum and basal pons. Like climbing fibres, they are excitatory, but they contrast sharply in their anatomical distribution and physiological properties. As each mossy fibre traverses the white matter, its branches diverge to enter several adjacent folia. Within each folium, these branches expand into grape-like synaptic terminals (mossy fibre rosettes) that occupy the centre of cerebellar glomeruli. Noradrenergic and serotoninergic fibres form a rich plexus in all layers of the cerebellar cortex. The aminergic fibres are fine and varicose and form extensive cortical plexuses; their release of noradrenaline (norepinephrine) and serotonin is assumed to be non-synaptic, and their effects are paracrine, involving volumes of tissue. The serotoninergic afferents of the cerebellum take their origin from neurones in the medullary reticular formation, other than the raphe nuclei. Cerebellar afferents have been traced from dopaminergic cells in the ventral tegmental area, and dopamine D2 and D3 receptors are present in the molecular layer. The connections of the cerebellum are organized in two perpendicular planes, corresponding to the planar organization of the cerebellar cortex. Efferent connections of the cortex are disposed in parasagittal sheets or bundles that connect longitudinal strips of Purkinje cells with specific cerebellar or vestibular nuclei. The climbing fibre afferents to a Purkinje cell zone from the inferior olive display a similar zonal disposition. Cerebellar output is organized in modules, with a module consisting of one or more Purkinje cell zones, their cerebellar or vestibular target nucleus and their olivocerebellar climbing fibre input. Modular function is determined by the brain stem projections of the cerebellar or vestibular target nucleus. Mossy fibre afferent systems from precerebellar nuclei in the spinal cord and the brain stem terminate in the granular layer of certain lobules in transversely oriented terminal fields. Parallel fibres cross and terminate on Purkinje cells belonging to several successive modules as they course through the molecular layer. Granule cell activity generates simple spikes, which resemble the response of other neurones in the brain, whereas activation by a climbing fibre produces a prolonged depolarization on which several spike-like waves are superimposed. Whereas the Purkinje cell may fire simple spikes at a rate of hundreds per second, complex spikes occur at very low frequencies, seldom more than three or four per second. Like Purkinje cells, Golgi cells have a rich dendritic tree that extends through the molecular layer. Unlike Purkinje cells, the Golgi cell dendrites are not restricted to a plane transverse to the folia, and their axons do not leave the cerebellar cortex. Golgi cells regulate firing by presynaptic inhibition of the mossy fibre afferents, so they act as a governor, or rate limiter, of Purkinje cell activity. Stellate and basket cells synapse directly on Purkinje cells and are powerful inhibitors of their activity. The efferent pathways of these regions monitor the activity in the corticospinal tract and in the subcortical motor systems descending from the vestibular nuclei and reticular formation. The inputs to the cerebellum and the outputs from it are organized according to the same somatotopic patterns, but the orientation of these patterns is reversed. The representation of the head is found principally in the simple lobule and caudally in a corresponding region of the posterior lobe. Vestibular connections of the cerebellum display a similar double representation in the most rostral lobules of the anterior lobe and far caudally in the vestibulocerebellum. The folium, tuber, uvula, tonsil and posterior biventral lobule all receive an almost pure pontine mossy fibre input. Climbing fibres from the inferior olive and mossy fibres from the basilar and tegmental pontine nuclei relay visual and acoustic information from the respective cerebral association areas and midbrain tectum to the folium and tuber that are thought to represent a vermal visual and acoustic area. The efferent connections of this area travel via the fastigial nucleus to gaze centres in the pons and midbrain. Her gait is broad based and unsteady, and she has impaired dexterity in the right hand, with virtually unintelligible handwriting and a prominent crescendo intention tremor on the right finger-to-nose test. Imaging demonstrates a large cystic lesion in the right cerebellar hemisphere, with compression of the fourth ventricle and secondary enlargement of the aqueduct and the lateral ventricles. At surgery, a well-defined mass is found at the margin of the cyst, a so-called mural nodule with the histological characteristic of a low-grade astrocytoma. Discussion: this patient demonstrates a cerebellar deficit ipsilateral to the cystic astrocytoma in the right cerebellar hemisphere, along with features of secondary hydrocephalus (headache, vomiting, blurring of vision) due to compression of the fourth ventricle.
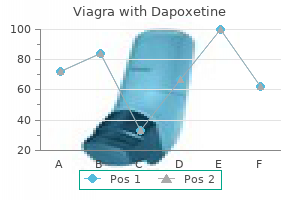
Discount viagra with dapoxetine 100/60 mg online
In front of the lamina terminalis diabetes and erectile dysfunction relationship viagra with dapoxetine 50/30mg amex, and almost coextensive with it, is the paraterminal gyrus, a narrow triangle of grey matter separated from the rest of the cortex by a shallow posterior paraolfactory sulcus. A short vertical sulcus, the anterior paraolfactory sulcus, may occur a little anterior to the paraterminal gyrus. The anterior region of the medial surface is divided into outer and inner zones by the curved cingulate sulcus, starting below the rostrum and passing first forward, then up and finally backward, conforming to the callosal curvature. Its posterior end turns up to the superomedial margin approximately 4 cm behind its midpoint and is posterior to the upper end of the central sulcus. The outer zone, except for its posterior extremity, is part of the frontal lobe; it is subdivided into anterior and posterior areas by a short sulcus that ascends from the cingulate sulcus above the midpoint of the corpus callosum. The larger anterior area is the medial frontal gyrus, and the posterior is the paracentral lobule. The superior end of the central sulcus usually invades the paracentral lobule posteriorly, and the precentral gyrus is continuous with the lobule. This area is concerned with movements of the contralateral lower limb and perineal region; clinical evidence suggests that it exercises voluntary control over defecation and micturition. Starting below the rostrum, this gyrus follows the callosal curve, separated by the callosal sulcus. It continues around the splenium to the inferior surface and then into the parahippocampal gyrus through the narrow isthmus. The posterior region of the medial surface is traversed by the parietooccipital and calcarine sulci. These two deep sulci converge anteriorly to meet a little posterior to the splenium. The parieto-occipital sulcus marks the boundary between the parietal and occipital lobes. It starts on the superomedial margin of the hemisphere approximately 5 cm anterior to the occipital pole, sloping down and slightly forward to the calcarine sulcus. Although usually restricted to the medial surface, its posterior end may reach the lateral surface. Directed anteriorly, it joins the parieto-occipital sulcus at an acute angle behind the splenium. The visual cortex lies above and below the posterior part of the calcarine sulcus, behind the junction with the parietooccipital sulcus. The calcarine is deep and produces an elevation, the calcar avis, in the wall of the posterior horn of the lateral ventricle. The area posterior to the upturned end of the cingulate sulcus, and anterior to the parieto-occipital sulcus, is the precuneus. It forms the medial surface of the parietal lobe with the part of the paracentral lobule behind the central sulcus. The medial surface of the occipital lobe is formed by the cuneus, a wedge of cortex bounded in front by the parieto-occipital sulcus, below by the calcarine sulcus and above by the superomedial margin. It is transversely concave and lies above the cribriform plate of the ethmoid, the orbital plate of the frontal and the lesser wing of the sphenoid. A rostrocaudal olfactory sulcus traverses the region near its medial margin, overlapped by the olfactory bulb and tract. The rest of this surface bears irregular orbital sulci, generally H-shaped, that divide it into the anterior, medial, posterior and lateral orbital gyri. Anteriorly, it may continue into the rhinal sulcus, but the two are usually separate. The rhinal sulcus (fissure) runs forward in the line of the collateral sulcus, separating the temporal pole from the hook-shaped uncus posteromedial to it. The occipitotemporal sulcus is parallel to the collateral sulcus and lateral to it. The lingual gyrus, between the calcarine and collateral sulci, passes into the parahippocampal gyrus. The parahippocampal gyrus begins at the isthmus, where it is continuous with the cingulate gyrus, and passes forward, medial to the collateral and rhinal sulci. Anteriorly, the parahippocampal gyrus continues into the uncus, its medial edge lying lateral to the midbrain. The uncus is the anterior end of the parahippocampal gyrus and is the posterolateral boundary of the anterior perforated substance. It is part of the piriform lobe of the olfactory system, which is phylogenetically one of the oldest parts of the cortex. The medial occipitotemporal gyrus extends from the occipital to the temporal poles. It is limited medially by the collateral and rhinal sulci and laterally by the occipitotemporal sulcus. The lateral occipitotemporal gyrus is continuous, around the inferolateral margin of the hemisphere, with the inferior temporal gyrus. The microscopic structure of the cerebral cortex is an intricate blend of nerve cells and fibres, neuroglia and blood vessels. The principal cell types are described first, followed by their laminar organization within the cortex. Non-pyramidal cells, also called stellate or granule cells, are divided into spiny and non-spiny neurones. The soma gives rise to a single thick apical dendrite and multiple basal dendrites. The apical dendrite ascends toward the cortical surface, tapering and branching, to end in a spray of terminal twigs in the most superficial lamina, the molecular layer. From the basal surface of the cell body, dendrites spread more horizontally, for distances up to 1 mm for the largest pyramidal cells. Like the apical dendrite, the basal dendrites branch profusely along their length. A single slender axon arises from the axon hillock, which is usually situated centrally on the basal surface of the pyramidal neurone. Ultimately, in the vast majority of (if not all) cases, the axon leaves the cortical grey matter to enter the white matter. They appear to use excitatory amino acids, either glutamate or aspartate, exclusively as their neurotransmitters. Several primary dendrites, profusely covered in spines, radiate for varying distances from the cell body. The right temporal pole has been displaced laterally to expose underlying structures. Shown from left to right are the Martinotti (M), neurogliaform (N), basket (B), horizontal (H), fusiform (F), stellate (S) and pyramidal (P) types of neurone. B, the most frequent types of neocortical neurone, showing typical connections with one another and with afferent fibres (blue). The right and left afferent fibres are association or corticocortical connections; the central afferent is a specific sensory fibre. Neurones are shown in their characteristic lamina, but many have somata in more than one layer. The smallest group comprises the heterogeneous non-spiny or sparsely spinous stellate cells. In morphological terms, this is not a single class of cell but a multitude of different forms, including basket, chandelier, double bouquet, neurogliaform, bipolar-fusiform and horizontal cells. Neurones with mainly horizontally dispersed axons include basket and horizontal cells. Basket cells have a short, vertical axon that rapidly divides into horizontal collaterals; these end in large terminal sprays synapsing with the somata and proximal dendrites of pyramidal cells. B, Isolated Golgi-stained neurones are prominent among the Nissl-stained cortical elements. They are small and fusiform, and their dendrites spread short distances in two opposite directions in lamina I. Their axons often stem from a dendrite, then divide into two branches that travel away from each other for great distances in the same layer.
Buy viagra with dapoxetine 50/30 mg on-line
In adults erectile dysfunction young age treatment purchase generic viagra with dapoxetine online, the weight ratio of cerebellum to cerebrum is approximately 1: 10; in infants, it is approximately 1: 20. The cerebellum is a central part of the major circuitry that links sensory to motor areas of the brain, and it is required for the coordination of fine movement. In health, it provides corrections during movement, which are the bases for precision and accuracy, and it is critically involved in motor learning and reflex modification. It receives sensory information through spinal, trigeminal and vestibulocerebellar pathways and, via the pontine nuclei, from the cerebral cortex and the tectum. Cerebellar output is mainly to those structures of the brain that control movement. The basic internal organization of the cerebellum is that of a superficial, highly convoluted cortex (a laminated sheet of neurones and supporting cells) overlying a dense core of white matter. The latter contains deep cerebellar nuclei, which give rise to the efferent cerebellar projections. Although the human cerebellum makes up approximately one-tenth of the entire brain by weight, the surface area of the cerebellar cortex, if unfolded, would be about half that of the cerebral cortex. The great majority of cerebellar neurones are small granule cells; they are so densely packed that the cerebellar cortex contains many more neurones than the cerebral cortex. Unlike the cerebral cortex, where a large number of diverse cell types are arranged differently in different regions, the cerebellar cortex contains a relatively small number of different cell types that are interconnected in a highly stereotypical way. Disease processes affecting the cerebellum or its connections lead to incoordination. Movements of the eyes, speech apparatus, individual limbs and balance are usually affected, which results in nystagmus, dysarthria, incoordination and ataxia. Although all these movements become defective in widespread disease of the cerebellum or its connections, topographical arrangements within the cerebellum lead to a variety of clinically recognizable disease patterns. Thus, in cerebellar hemisphere disease, the ipsilateral limbs show rhythmical tremor during movement but not at rest. The tremor increases as the target is approached, so reaching and accurate movements of the arm are especially difficult. Diseases that affect the ascending spinocerebellar pathways or the midline vermis have a disproportionate effect on axial structures, leading to severe loss of balance. Lesions of outflow tracts in the superior cerebellar peduncles result in a wide-amplitude, severely disabling, proximal tremor that interferes with all movements and may even disturb posture, leading to rhythmic oscillations of the head or trunk so that the patient is unable to stand or sit without support. However, although cerebellar lesions may initially cause profound motor impairment, a considerable degree of recovery is possible. There are clinical reports that the initial symptoms of large cerebellar lesions (caused by trauma or surgical excision) have improved progressively over time. Although the basic structure of the cerebellum and its importance for normal movement have long been recognized, many of the details of how it functions remain obscure. The main goal of this chapter is to describe the known structure and connections of the cerebellum. The transverse sinus borders the cerebellum at the point where the superior and inferior surfaces meet. The inferior surface is characterized by a massive enlargement of the cerebellar hemispheres, which extends medially to overlie some of the vermis. Posteriorly, the hemispheres are separated by a deep vallecula, which contains the dural falx cerebelli. It forms the roof of the fourth ventricle and the lateral recesses on each side of it, while the cerebellar peduncles define the diamond shape of the ventricle when viewed from behind. Anterolaterally, the cerebellum lies against the posterior surface of the petrous part of the temporal bone. The cerebellar surface is divided by numerous curved transverse fissures that separate its folia and give it a laminated appearance. One conspicuous fissure, the horizontal fissure, extends around the dorsolateral border of each hemisphere from the middle cerebellar peduncle to the vallecula, separating the superior and inferior surfaces. Although the horizontal fissure is prominent, it appears relatively late in embryological development and does not mark the boundary between major functional subdivisions of the cortex. The deepest fissure in the vermis is the primary fissure, which curves ventrolaterally around the superior surface of the cerebellum to meet the horizontal fissures. It appears early in embryological development and marks the boundary between the anterior and posterior lobes. Because the cerebellar cortex has a roughly spherical shape, the true relations between its parts can sometimes be obscured. Thus, the most anterior lobule of the cerebellar vermis, the lingula, lies very close to the most posterior lobule, the nodule. The lobules of the superior vermis that belong to the anterior lobe are the lingula, central lobule and culmen. The central lobule and culmen are continuous bilaterally with an adjoining wing (ala) in each hemisphere. The culmen (with attached anterior quadrangular lobules) lies between the preculminary and primary fissures. Between the primary and horizontal fissures are the simple lobule (with attached posterior quadrangular lobules) and the folium (with attached superior semilunar lobules). From the back forward, the inferior vermis is divided into the tuber, pyramis, uvula and nodule, in that order. The tuber is continuous laterally with the inferior semilunar lobules and separated from the pyramis by the lunogracile fissure. The pyramis and attached biventral lobules (containing an intrabiventral fissure) are separated from the uvula and attached cerebellar tonsils by the secondary fissure. Behind the uvula, and separated from it by the median part of the posterolateral fissure, is the nodule. The tonsils are roughly spherical and overhang the foramen magnum on each side of the medulla oblongata. The nodule and attached flocculi constitute a separate flocculonodular lobe that is separated from the uvula and tonsils by the deep posterolateral fissure. This lobe is richly interconnected with the vestibular nucleus, which is located at the lateral margin of the fourth ventricle. The superior surface of the cerebellum, which would constitute the anterior part of the unrolled cerebellar cortex, is relatively flat. The paramedian sulci are shallow, and the borders between vermis and hemispheres are indicated by kinks in the transverse fissures. The superior surface adjoins the tentorium cerebelli and projects the cerebellum is divided functionally into a body, with inputs mainly from the spinal cord and pontine nuclei, and a flocculonodular lobe, which has strong afferent and efferent connections with the vestibular nuclei. The body is subdivided into a series of regions dominated by their spinal or pontine inputs. The anterior lobe, simple lobule, pyramis and biventral lobules are the main recipients of spinal and trigeminal cerebellar afferents. The mediolateral subdivision of the cerebellum into vermis and hemispheres represents a functional subdivision that is closely related to its output. In mammals, a great increase in the size of the cerebellar hemispheres parallels the development of the cerebral cortex and reflects the importance of the corticopontocerebellar input and of the efferent projections of the cerebellar hemispheres (through the dentate and interposed cerebellar nuclei and the thalamus) to the cerebral cortex. Cerebellar Peduncles passing dorsal to the superior cerebellar peduncle, enter the brain stem as the uncinate fasciculus at the border of the juxtarestiform and restiform bodies. The superior cerebellar peduncle contains all the efferent fibres from the dentate, emboliform and globose nuclei and a small fascicle from the fastigial nucleus. It decussates with its opposite number in the caudal mesencephalon, on its way to synapse in the contralateral red nucleus and thalamus. The anterior spinocerebellar tract reaches the upper part of the pontine tegmentum before looping down within this peduncle to join the spinocerebellar fibres entering through the restiform body. The white core of the cerebellum branches in diverging medullary laminae, which occupy the central part of the lobules and are covered by the cerebellar cortex. In a sagittal section through the cerebellum, the highly branched pattern of medullary laminae is known as the arbor vitae. The white core consists of the efferents (Purkinje cell axons) and afferents of the cerebellar cortex.
Generic viagra with dapoxetine 100/60mg on line
The cell layers deep to the parasubiculum are indistinguishable from the deep layers of the entorhinal cortex erectile dysfunction diabetes type 2 treatment buy 50/30 mg viagra with dapoxetine with mastercard. The more primitive levels of the entorhinal cortex (below the amygdala) receive projections from the olfactory bulb. The entorhinal cortex is divisible into six layers and is quite distinct from other neocortical regions. They form small bumps on the surface of the brain that can be seen by the naked eye (verrucae hippocampae) and indicate the boundaries of the entorhinal cortex. Its cells continue around the angular bundle (subcortical white matter deep to the subicular complex, made up largely of perforant path axons) to lie beneath the pre- and parasubiculum. Dentate gyrus, pink; hippocampus proper (cornu ammonis), yellow; areas of the subicular complex, green; entorhinal cortex, blue. Enkephalin, or a related peptide, may be present in fibres arising in the entorhinal cortex. There is a dense plexus of somatostatin-immunoreactive fibres in the molecular layer of the dentate gyrus and also in the stratum lacunosummoleculare of the hippocampus. The polymorphic layer of the dentate gyrus, stratum oriens of the hippocampus and deep layers of the entorhinal cortex all contain somatostatin-immunoreactive neurones. The axons terminate in the outer two thirds of the molecular layer of the dentate gyrus, on the dendritic spines of granule cells. The subiculum, rather than the hippocampus, projects to the mammillary complex, whereas the hippocampus gives rise principally to efferents destined for the septal complex. The medial septal complex and the supramammillary area of the posterior hypothalamus are the two major sources of subcortical afferents to the hippocampal formation. Cell somata, dendrites and axons of the pyramidal neurones of the cornu ammonis are yellow; the axons form the efferent hippocampal fibres of the alveus and fimbria. Afferent fibres to the cornu ammonis from the fimbria are purple; afferents from the entorhinal cortex via the perforant path are blue; basket neurones are black; neurones of the dentate gyrus and their axons, which form the mossy fibres of the hippocampus, are magenta; and subicular efferents to the fornix via the alveus are green. The noradrenergic and serotoninergic projections reach all hippocampal fields but are especially dense in the dentate gyrus. The projections from the septal complex arise in the medial septal and vertical limb nuclei of the diagonal band. They travel via the dorsal fornix, fimbria, and supracallosal striae and take a ventral route through the amygdaloid complex. Neurones in the supramammillary area also provide significant innervation of the hippocampal formation. All divisions of the anterior thalamic nuclear complex and the associated lateral dorsal nucleus project to the hippocampal formation and are directed predominantly to the subicular complex. Some midline thalamic nuclei, particularly the parataenial, central medial and reuniens nuclei, also project to the hippocampal formation, especially to the entorhinal cortex. Neurones in the subicular complex and entorhinal cortex give rise to projections to the nucleus accumbens and to parts of the caudate nucleus and putamen. The subicular complex gives rise to the major postcommissural fibre system of the fornix. Both the subiculum and the presubiculum provide the major extrinsic input to the mammillary complex. Both the lateral and the medial mammillary nuclei receive afferents from the subicular complex. The subicular complex receives direct cortical inputs, such as from the temporal polar cortex, perirhinal cortex, parahippocampal gyrus, superior temporal gyrus and dorsolateral prefrontal cortex. The entorhinal cortex projects to the perirhinal cortex as well as to the temporal polar cortex and caudal parahippocampal and cingulate gyri. In monkeys, the subicular complex also projects to a number of cortical areas, including the perirhinal cortex, parahippocampal gyrus, caudal cingulate gyrus, and medial frontal and medial orbitofrontal cortices. Projections from the lateral septum run to the medial and lateral preoptic areas, anterior hypothalamus, and supramammillary and midbrain ventral tegmental area via the medial forebrain bundle. There is also a projection to the medial habenular nucleus and to some midline thalamic nuclei via the stria medullaris thalami, which runs on the dorsomedial wall of the third ventricle. The projections from the habenula via the fasciculus retroflexus to the interpeduncular nucleus and adjacent ventral tegmental area in the midbrain provide a route through which forebrain limbic structures can influence midbrain nuclear groups. The amygdaloid nuclear complex is made up of lateral, central and basal nuclei that lie in the dorsomedial temporal pole, anterior to the hippocampus, and close to the tail of the caudate nucleus. Collectively, the nuclei form the ventral, superior and medial walls of the tip of the inferior horn of the lateral ventricle. The amygdala is partly continuous above with the inferomedial margin of the claustrum. Fibres of the external capsule and substriatal grey matter, including the cholinergic magnocellular nucleus basalis (of Meynert), incompletely separate it from the putamen and globus pallidus. The basal nucleus is commonly divided into a dorsal magnocellular basal nucleus, an intermediate parvocellular basal nucleus and a ventral band of darkly staining cells usually referred to as the paralaminar basal nucleus because it borders the white matter ventral to the amygdaloid complex. The lateral and basal nuclei are often referred to collectively as the basolateral area (nuclear group) of the amygdaloid complex. Its upper portion corresponds largely to the bilateral laminae of fibres, sparse grey matter and neuroglia known as the septum pellucidum, which separates the lateral ventricles. Below this, the septal region is made up of four main nuclear groups: dorsal, ventral, medial and caudal. The dorsal group is essentially the dorsal septal nucleus, the ventral group consists of the lateral septal nucleus, the medial group contains the medial septal nucleus and the nucleus of the diagonal band of Broca and the caudal group contains the fimbrial and triangular septal nuclei. The major afferents to the region terminate primarily in the lateral septal nucleus. There are also afferents arising from the preoptic area; anterior, paraventricular and ventromedial hypothalamic nuclei; and lateral hypothalamic area. The earliest morphological changes are found in the medial temporal lobe and in the basal nucleus (of Meynert), with secondary loss of acetylcholine transferase, especially in the neocortex, reflecting the degeneration of cholinergic projections from the basal nucleus. A 64-year-old man is brought for evaluation by his family because of impaired memory noted in the past 6 months. He has been unable to keep up with the demands of his business, has made a number of unfortunate decisions reflecting faulty judgment and has found it necessary to turn control of the family finances over to his wife. He has become increasingly apathetic and withdrawn, removing himself from his customary active social life. He has impaired memory for both recent and remote events, can no longer carry out simple arithmetic calculations, has a shortened attention span and is unable to explain proverbs or similarities in an abstract manner. With the exception of a mild, predominantly dysnomic form of aphasia and the occasional appearance of Parkinsonian signs, such as bradykinesia and rigidity, the remainder of the neurological examination is normal. Ultimately, much of the cerebral grey matter is involved, with typical neuropathological alterations and neurofibrillary changes reflecting intracellular accumulation of. Although it lacks a laminar structure, it has direct, often reciprocal, connections with adjacent temporal and other areas of cortex, and it projects to the motor or premotor cortex. It receives a direct cholinergic and non-cholinergic input from the magnocellular corticopetal system in the basal forebrain and has reciprocal connections with the mediodorsal thalamus. The distribution of small peptidergic neurones in the basolateral nuclear complex. Projection neurones from this part of the amygdala appear to utilize, at least in part, the excitatory amino acids glutamate and aspartate as transmitters. Moreover, they project to the ventral striatum rather than to hypothalamic and brain stem sites. Thus, it may be appropriate to consider this part of the amygdaloid complex as a polymodal cortex-like area that is separated from the cerebral cortex by fibres of the external capsule. The central nucleus is present through the caudal half of the amygdaloid complex, lying dorsomedial to the basal nucleus. The medial part, which contains larger cells than the lateral part, resembles the adjacent putamen. The medial and central nuclei appear to have an extension across the basal forebrain, as well as within the stria terminalis, which merges with the bed nucleus of the stria terminalis. It can be considered a macrostructure formed by the centromedial amygdaloid complex (medial nucleus, medial and lateral parts of the central nucleus), the medial bed nucleus of the stria terminalis and the cell columns that traverse the sublenticular substantia innominata, which lies between them.
Diseases
- Multiple sclerosis ichthyosis factor VIII deficiency
- Cormier Rustin Munnich syndrome
- Angioneurotic edema hereditary due to C1 esterase deficiency
- Myasthenia, familial
- Sacral hemangiomas multiple congenital abnormalities
- Narcissistic personality disorder
Discount viagra with dapoxetine 50/30mg online
Oncologic beads (chemotherapeutic-loaded drug-eluting beads or yttriumloaded radioembolic beads) may offer treatment options erectile dysfunction treatment homeopathy buy 100/60 mg viagra with dapoxetine. Preoperatively before nephrectomy, inexpensive Gelfoam and coils may be appropriate. In a nonoperative situation, particles and liquids will have the best outcome, but be cautious of shunting suspected on preembolization image. Together, these embolic agents should reduce the risk of further gastric variceal hemorrhage. Glue and Onyx Embolization of Extrasegmental Bile Duct Leak (Diagnostic Sinogram) Glue and Onyx Embolization of Extrasegmental Bile Duct Leak (During Embolization) (Left) Embolics have nonvascular utility [e. Contrast injected through a surgical drain tracks into a biliary duct, inadvertently surgically excluded from the biliary system. Slow Onyx injection, followed by rapid "glue" injection and access removal, successfully halted the leak. Covered Stents (Stent-Grafts) Stent Deployment (Photographic Appearance) (Left) Photograph of a selfexpanding stent shows the tapered catheter tip, the partially expanded stent, and the retractable sheath, which constrains the stent until it is unsheathed within the target vessel. Basavarajaiah S et al: Treatment of drug-eluting stent restenosis: comparison between drug-eluting balloon versus second-generation drug-eluting stents from a retrospective observational study. Subclavian Steal (Left Subclavian Arteriogram) Subclavian Steal (Post Stent Deployment) (Left) Selective arteriogram of the left subclavian artery reveals a partially obstructive linear defect near the subclavian artery origin. Hepatic Artery Aneurysm (Aortogram) Hepatic Artery Aneurysm (Stent Deployment) (Left) After surgery failed to treat, it was decided that the aneurysm would be excluded with a covered stent (off-label usage). Contrast injected through the guide sheath outlined the aneurysm during stent deployment. Healthy vascular tissue adjacent to the diseased segment is critical to covered stent exclusion of an aneurysm. The stentgraft excludes the aneurysm from circulation, eliminating the potential for aneurysm thrombosis or distal embolization. There are proximal and distal radiopaque markers that denote the stent margins for precise deployment. Note that the balloon expands first at the proximal and distal end of the stent, preventing the stent from migrating as it is deployed. Once dilated, the high intrinsic radial force of the stent maintains patency of the vessel. Renal Artery Balloon-Mounted Stent Deployment (Diagnostic Arteriogram) Renal Artery Balloon-Mounted Stent Deployment (Diagnostic Arteriogram) (Left) A diagnostic right renal arteriogram was obtained via a reverse curve catheter selectively engaged at the arterial takeoff. Emergent celiac arteriography revealed a bleeding proper hepatic artery pseudoaneurysm. Arteriobiliary Fistula (Placement of Balloon-Mounted Covered Stent) (Left) Due to the close proximity of the bleed to the adjacent takeoff arteries and the short available landing zone, we opted to maximize stent position by placing a balloon-mounted stent. The bare metal portion of the stent is placed within the portal vein, allowing antegrade flow to continue unobstructed through the portal vein. Central Venous Occlusion (Prestent) Central Venous Occlusion (Poststent) (Left) A patient who had been receiving hemodialysis via a left arm graft complained of recently increased left arm swelling. Venous In-Stent Restenosis (Fractured Stent, Subclavian Vein) Venous In-Stent Restenosis (Hemodialysis Fistula Outflow) (Left) Stenting of the subclavian vein often leads to stent fracture and resultant instent stenosis. In this case, stenosis was associated with a high-flow hemodialysis fistula and rapidly reoccurred despite repeated angioplasty. Splenic artery aneurysms are 4x more common in women than men, particularly if there is a history of multiple pregnancies. Treatment options that were considered included placing a covered stent across the aneurysm neck to exclude the aneurysm vs. Contrast has been injected via the microcatheter, opacifying the aneurysm and confirming the catheter tip position. The stent prevents the coils from prolapsing into, and occluding, the splenic artery. Stent-Assisted Coil Embolization (Coil Deployment into Aneurysm) 72 Stents: Vascular General Principles In-Stent Intimal Hyperplasia (Diagnostic Aortogram) In-Stent Intimal Hyperplasia (Post Deployment of Balloon-Mounted Stent) (Left) High-grade proximal right renal artery stenosis is seen in a patient with poorly controlled hypertension and mild renal insufficiency. In-Stent Intimal Hyperplasia (10-Month Follow-Up) In-Stent Intimal Hyperplasia (Restent) (Left) Hypertension was initially well controlled after stent placement, but the patient presented 10 months later with recurrent hypertension. Repeat angiography revealed a narrowed lumen within the stent with a normal-caliber renal artery distally, findings consistent with instent intimal hyperplasia. Angiography is often necessary to diagnose intimal hyperplasia when there is clinical concern. It has many applications, including treatment of colonic, biliary, and vascular obstructions. Additional important but offlabel uses exist, including emergent treatment of arterial bleeding when exact stent location is necessary. The stent contains covered (above) and fenestrated (below) segments separated by a radiopaque band. Ureteral Stent Colorectal Stent (Left) A double J ureteral stent has been deployed in a patient with ureteral obstruction from peritoneal carcinomatosis. The proximal pigtail is in the renal pelvis, and the distal pigtail in the bladder. Subsequently, this cholangiogram revealed an obstructing stone in the left main biliary duct. This particular stent may be manipulated after deployment by grasping and pulling on the blue radiopaque band. A stent can be introduced over the guidewire and any debris aspirated via the sheath. Contrast injection via the sheath is used to confirm an appropriate stent position prior to deployment. A guidewire placed via the sheath is advanced across the stenosis while the sheath is aspirated. Contrast injection shows the guidewire crossing the stenosis and a large calcified plaque at the proximal end of the stenosis. A 2nd filling defect, more cephalad in the popliteal artery, has not yet been captured in the device. These were isolated varices that were located mainly in the gastric fundus with no obvious esophageal varices seen. A balloon occlusion catheter was advanced through the left renal vein, and the tip was placed in the gastrorenal shunt. Various sclerosing agents can be used, including ethanolamine oleate iopamidol, sodium tetradecyl sulfate, and polidocanol, in either liquid or foam consistency. N-butylcyanoacrylate (glue) and absolute ethanol have also been used as liquid sclerosants. The access needle is inserted perpendicular to the vessel and parallel to the plane of scan. An access needle is inserted from the side of the vessel and seen in entire length. An access needle is inserted parallel to the vessel and perpendicular to the plane of scan. An access needle is inserted parallel to the vessel and parallel to the plane of scan. The access needle is inserted parallel to the plane of scan over the vessel and seen in entire length. Continued pressure while advancing the needle allows puncture of the tented wall and lumen entry. Intraluminal position of the needle tip is confirmed with blood return at the needle hub. The inner dilator of the microaccess sheath and the microwire are removed, and an 0. Optimal location of the femoral venous access is inferior to the inguinal ligament.
Generic viagra with dapoxetine 100/60 mg on-line
On examination he has mild atrophy of all anterior thigh muscles on the right erectile dysfunction rap trusted 100/60 mg viagra with dapoxetine, with moderate weakness in the same muscles. Hamstring, hip flexors and extensors and all distal muscles exhibit normal strength. On sensory examination he has mild bilateral distal sensory loss, along with decreased Achilles reflexes. Discussion: Diabetic amyotrophy (proximal diabetic neuropathy) often has an acute or subacute onset, affecting the anterior thigh muscles more than hip, posterior thigh or distal leg musculature. Ischaemic injury to the proximal lumbosacral plexus, probably due to a vasculitic process, is the most likely cause. S2, S3 L4, L5, S1, S2, S3 Sciatic Nerve the sciatic nerve is 2 cm wide at its origin and is the thickest nerve in the body. It leaves the pelvis via the greater sciatic foramen below piriformis and descends between the greater trochanter and the ischial tuberosity and along the back of the thigh, dividing into the tibial and common peroneal (fibular) nerves at a varying level proximal to the knee. Superiorly, it lies deep to gluteus maximus, resting first on the posterior ischial surface with the nerve to quadratus femoris between them. It then crosses posterior to obturator internus, the gemelli and quadratus femoris, separated by the last from obturator externus and the hip joint. It is accompanied medially by the posterior femoral cutaneous nerve and the inferior gluteal artery. More distally, it lies behind adductor magnus and is crossed posteriorly by the long head of biceps femoris. It corresponds to a line drawn from just medial to the midpoint between the ischial tuberosity and the greater trochanter to the apex of the popliteal fossa. Articular branches arise proximally to supply the hip joint through its posterior capsule; these are sometimes derived directly from the sacral plexus. Muscular branches are distributed to biceps femoris, semitendinosus, semimembranosus and the ischial part of adductor magnus. The point of division of the sciatic nerve into its major components (tibial and common peroneal) is very variable. A common site is at the junction of the middle and lower thirds of the thigh, near the apex of the popliteal fossa. Anococcygeal nerves To levator ani, coccygeus and sphincter ani externus Pudendal nerve. The contribution from S2 to the pelvic splanchnic nerves has been cut before it joins those from S3 and S4. Lesions of the Sciatic Nerve the sciatic nerve supplies the knee flexors and all the muscles below the knee, so that a complete palsy of the sciatic nerve results in a flail foot and severe difficulty walking. As it leaves the pelvis it passes either behind piriformis or sometimes through the muscle, and at that point it may become entrapped (piriformis syndrome); this is a common anatomical variant, but an extremely rare entrapment neuropathy. The safe zone for deep intramuscular injections is the upper outer quadrant of the buttock. Perhaps safer still is to inject into quadriceps, although this can produce other problems such as haemorrhage, leading to contracture of the muscle, which limits knee motion. Sciatic nerve palsy occurs after total hip replacement or similar surgery in approximately 1% of cases. Haematoma is characterized by the development of severe pain in the immediate postoperative period. Early surgical exploration and evacuation of haematoma can reverse the nerve lesion. Unfortunately, the other causes may not be treatable; however, the majority are temporary. For some reason, possibly anatomical, the common peroneal part is usually affected alone. The tibial nerve, the larger sciatic component, is derived from the ventral branches (anterior division) of the fourth and fifth lumbar and first to third sacral ventral rami. It descends along the back of the thigh and popliteal fossa to the distal border of popliteus. It then passes anterior to the arch of soleus with the popliteal artery and continues into the leg. In the thigh it is overlapped proximally by the hamstring muscles, but it becomes more superficial in the popliteal fossa, where it is lateral to the popliteal vessels. At the level of the knee the tibial nerve becomes superficial to the popliteal vessels and crosses to the medial side of the artery. In the distal popliteal fossa it is overlapped by the junction of the two heads of gastrocnemius. In the leg the tibial nerve descends with the posterior tibial vessels to lie between the heel and the medial malleolus. Proximally, it is deep to soleus and gastrocnemius, but in its distal third it is covered only by skin and fasciae, overlapped sometimes by flexor hallucis longus. At first medial to the posterior tibial vessels, it crosses behind them and descends lateral to them until it bifurcates. It lies on tibialis posterior for most of its course except distally, where it adjoins the posterior surface of the tibia. The tibial nerve ends under the flexor retinaculum by dividing into the medial and lateral plantar nerves. The branches of the tibial nerve are articular, muscular, sural, medial calcaneal and medial and lateral plantar. Articular Branches - Articular branches accompany the superior, inferior medial and middle genicular arteries to the knee joint. They form a plexus with a branch from the obturator nerve and supply the oblique posterior ligament. The branches accompanying the superior and inferior genicular arteries also supply the medial part of the capsule. Muscular Branches - Proximal muscular branches arise between the heads of gastrocnemius and supply gastrocnemius, plantaris, soleus and popliteus. The pain began suddenly 2 days ago when he bent over to pick something up off the floor. He cannot stand erect due to increasing pain and is most comfortable leaning to the left. He also observes tingling discomfort in the left leg involving the sole, lateral foot and fifth toe. He is very tender on percussion over the lumbar spine and exhibits moderate paravertebral muscle spasm. Discussion: Sciatica is a term used to describe pain radiating down the leg, with or without back pain. L5 and S1 roots are most commonly compressed; both roots can be involved in dorsolateral or central disc herniation, with the disc compressing the roots in the lateral recess. A large and more lateral herniation may result in only a single root being compressed, usually within the intervertebral foramen. Owing to mixed root innervation to lower extremity muscles, motor and sensory symptoms and signs do not always precisely match the root injured. Sensory loss in the foot (lateral foot and fifth toe involvement) indicates S1 localization. Straight leg raising increases symptoms by stretching the roots already compromised. Loss of the Achilles reflex with a normal patellar reflex is typical of an S1 root lesion. The branch to popliteus descends obliquely across the popliteal vessels, curling around the distal border of the muscle to its anterior surface. It also supplies tibialis posterior, the proximal tibiofibular joint and the tibia and gives off an interosseous branch that descends near the fibula to reach the distal tibiofibular joint. Muscular branches in the leg, either independently or by a common trunk, supply soleus (on its deep surface), tibialis posterior, flexor digitorum longus. The course of the common peroneal nerve can be indicated by a line from the apex of the popliteal fossa, passing distally, medial to the biceps tendon, to the back of the head of the fibula, where the nerve can be rolled against the bone. Two accompany the superior and inferior lateral genicular arteries and may arise in common. The third, the recurrent articular nerve, arises near the termination of the common peroneal nerve. It ascends with the anterior recurrent tibial artery through tibialis anterior and supplies the anterolateral part of the knee joint capsule and the proximal tibiofibular joint. Cutaneous Branches - the two cutaneous branches, often from a common trunk, are the lateral sural and sural communicating nerves.
Cheap viagra with dapoxetine line
Most pathways are multisynaptic causes to erectile dysfunction order viagra with dapoxetine 50/30mg online, which means that the majority of synapses on any hypothalamic neurone are derived from hypothalamic interneurones. Efferent neural projections are reciprocal to most of these sources; in particular, they impinge on and control the central origins of autonomic nerve fibres. The hypothalamus therefore exerts control via the autonomic and endocrine systems and through its connections to the telencephalon. The hypothalamus receives visceral, gustatory and somatic sensory information from the spinal cord and brain stem. It receives largely polysynaptic projections from the nucleus tractus solitarius, probably directly and indirectly via the parabrachial nucleus and medullary noradrenergic cell groups Efferent Connections Afferent Connections Hypothalamic efferents include reciprocal paths to the limbic system, descending polysynaptic paths to autonomic and somatic motor neurones and neural and neurovascular links with the pituitary. Septal areas and the amygdaloid complex have reciprocal hypothalamic connections along the paths described earlier. The medial preoptic and anterior hypothalamic areas give short projections to nearby hypothalamic groups. The ventromedial nucleus has more extensive projections that pass via the medial forebrain bundle to the bed nucleus of the stria terminalis, basal nucleus of Meynert, central nucleus of the amygdala and midbrain reticular formation. Some tuberal and posterior lateral hypothalamic neurones project directly to the entire neocortex and appear to be essential for maintaining cortical arousal, but the topography of these projections is unclear. She also reports difficulties with memory, concentration and attention and diffuse headaches in association with sleepiness. In addition, she reports automatisms, wherein she types illegibly on the computer as she falls asleep. Upon questioning, she reports vivid dreams during sleep, usually with a threatening content and associated with an inability to move to extricate herself from the imagined danger. She also recalls rare episodes of cataplexy-the sudden onset of weakness in her facial muscles after laughter or anger. On one occasion, she collapsed in a store when she lost all motor ability in both legs for a few minutes; she was rushed to the emergency room and released within a few hours, with no positive findings. She naps for 2 hours a day on weekends, finding naps to be refreshing; naps are associated with dreams. Past medical and psychiatric histories are negative, and she takes no medications. She consumes two caffeinated beverages in the morning, which allows her to function at a nominal cognitive level in the morning. Multiple sleep latency testing performed during the day following the nocturnal polysomnogram reveals a mean sleep latency of 2. This pattern of projections from the hypocretin neurones is thought to play an important role in arousal and maintenance of the awake state. Hypothalamic neurones projecting to autonomic neurones are found in the paraventricular nucleus (oxytocin and vasopressin neurones), perifornical and dorsomedial nuclei (atrial natriuretic peptide neurones), lateral hypothalamic area (-melanocyte-stimulating hormone neurones) and zona incerta (dopamine neurones). These fibres run through the medial forebrain bundle into the tegmentum, ventrolateral medulla and dorsal lateral funiculus of the spinal cord. In the brain stem, fibres innervate the parabrachial nucleus, nucleus ambiguus, nucleus of the solitary tract and dorsal motor nucleus of the vagus. In the spinal cord, they end on sympathetic and parasympathetic preganglionic neurones in the intermediolateral column. Both oxytocin- and vasopressin-containing fibres can be traced to the most caudal spinal autonomic neurones. The medial mammillary nucleus gives rise to a large ascending fibre bundle that diverges into mammillothalamic and mammillotegmental tracts. The mammillothalamic tract ascends through the lateral hypothalamus to reach the anterior thalamic nuclei, where massive projections radiate to the cingulate gyrus. The mammillotegmental tract curves inferiorly into the midbrain, ventral to the medial longitudinal fasciculus, and is distributed to the tegmental reticular nuclei. The pituitary gland, or hypophysis cerebri, is a reddish grey ovoid body approximately 12 mm in transverse diameter and 8 mm in anteroposterior diameter, weighing approximately 500 mg. It is continuous with the infundibulum, a hollow, conical inferior process from the tuber cinereum of the hypothalamus. It lies within the pituitary fossa of the Pituitary Gland sphenoid bone, where it is covered superiorly by a circular diaphragma sellae of dura mater. The latter is pierced centrally by an aperture for the infundibulum and separates the anterior superior aspect of the pituitary from the optic chiasma. Inferiorly, it is separated from the floor of the pituitary fossa by a venous sinus that communicates with the circular sinus. The pituitary has two major parts-neurohypophysis and adenohypophysis- which differ in their origin, structure and function. The infundibulum has a central infundibular stem that contains neural hypophysial connections and is continuous with the median eminence of the tuber cinereum. Thus, the neurohypophysis includes the median eminence, infundibular stem and neural lobe or pars posterior. Surrounding the infundibular stem is the pars tuberalis, a component of the adenohypophysis. Although usually obliterated in childhood, remnants may persist in the form of cystic cavities near the adenoneurohypophysial frontier, sometimes invading the neural lobe. It may be partially displaced into the neural lobe, so it has been included in the anterior and posterior parts by different observers. Apart from this equivocation, which is of little significance in view of the exiguous status of 272 Chapter 15 / Diencephalon Anterior commissure Anterior cerebral artery Tuber cinereum Posterior cerebral artery Oculomotor nerve Trochlear nerve Superior cerebellar artery Lamina terminalis Optic recess Optic chiasma Optic nerve Infundibular recess Arachnoid Posterior communicating artery Posterior lobe of hypophysis cerebri Basilar artery Sphenoidal sinus Anterior lobe of hypophysis cerebri. When the associated infundibular parts continuous with these lobes are included, the names adenohypophysis and neurohypophysis become appropriate and are used here as follows: neurohypophysis includes the pars posterior (pars nervosa, posterior or neural lobe), infundibular stem and median eminence; adenohypophysis includes the pars anterior (pars distalis or glandularis), pars intermedia and pars tuberalis. Neurohypophysis In early fetal life, the neurohypophysis contains a cavity continuous with the third ventricle. They form the neurosecretory hypothalamohypophysial tract and terminate near the sinusoids of the posterior lobe. Some smaller parvocellular neurones in the periventricular zone have shorter axons and end in the median eminence and infundibular stem among the superior capillary beds of the venous portal circulation. These small neurones produce releasing and inhibitory hormones, which control the secretory activities of the adenohypophysis via its portal blood supply. The neurohormones stored in the main part of the neurohypophysis are vasopressin (antidiuretic hormone), which controls reabsorption of water by renal tubules, and oxytocin, which promotes the contraction of uterine smooth muscle in childbirth and the ejection of milk from the breast during lactation. Storage granules containing active hormone polypeptides bound to a transport glycoprotein, neurophysin, pass down axons from their site of synthesis in the neuronal somata. The granules are seen as swellings along the axons and at their terminals, which can reach the size of erythrocytes. Near the posterior lobe, astrocytes are replaced by pituicytes, which constitute most of the nonexcitable tissue in the neurohypophysis. Pituicytes are dendritic neuroglial cells of variable appearance, often with long processes running parallel to adjacent axons. Typically, their cytoplasmic processes end on the walls of capillaries and sinusoids between nerve terminals. Although they are close to the walls of sinusoids, they remain separated from them by two basal laminae-one around the nerve endings, and the other underlying the fenestrated endothelial cells. The epithelial endocrine cells, which secrete the different adenohypophysial hormones, are distinguished in part by their differing affinities for acidic and basic dyes. Cells staining strongly are described as chromophils, and those with low affinity for dyes are chromophobes. Chromophils that stain strongly with acidic dyes are classed as acidophils, whereas basophils stain strongly with basic dyes; the latter are more prevalent in the central part of the gland. Chromophobes are thought to be quiescent or degranulated chromophils or immature precursor cells; they constitute up to one half the cells of the adenohypophysis. Neurones that secrete the peptides and amines that control the anterior lobe are widely distributed within the hypothalamus.
50/30 mg viagra with dapoxetine with visa
He associated the use of two different statins with muscle pains in the legs and has stopped them; he is unsure of his untreated cholesterol level erectile dysfunction pump how do they work order viagra with dapoxetine 100/60mg. This is an excellent topic for discussion and it is likely the examiners would be keen to pursue this problem. Consider aspirin sensitivity; however, he takes regular aspirin without difficulty. Would postural drainage, a flutter valve or physiotherapy be helpful for his productive cough Is candidate happy with the current management of follow-up of the two malignancies His gut problems began in 1996 with bloody diarrhoea and a diagnosis of inflammatory bowel disease. Biological agents were not available at that time and poor control of his symptoms led to a proctocolectomy and the need for an ileostomy drainage bag. He had further surgery with relocation of his stoma in 2006 because of a parastomal infection. In 2009 he had further surgery to relieve adhesions, which was largely successful. An immunoglobulin deficiency was diagnosed after investigations for failure of his wound to heal. He had two myocardial infarctions in 1997 and then a stent to the right coronary artery in 1998. The recurrent infection with bowel organisms and curious photographic record of the wound might make you think about a factitious cause. Possible ischaemic heart disease would lead to a standard spiel about risk factor tp control. The stoma appeared satisfactory and the abdominal examination was otherwise t/i nt failure. No signs of heart na l-m Examination ed ic There was a history of hyperlipidaemia and hypertension, currently treated with atorvastatin 10 mg, and he is not currently on antihypertensives. He takes clopidogrel rather than aspirin because of gastrointestinal sideeffects associated with aspirin. He has had a diagnosis of obstructive sleep apnoea; this appears to be mild and is not being treated. You may be keen to wheel out your usual diabetes presentation, but you may not be allowed to by the examiners. There may be time for only a brief discussion of the opioid problem with the usual discussion about referral to the pain management clinic. In these videos, typical long cases are discussed by the authors and then presented by a candidate. The written stem is given to the candidate or displayed on the door 1 or 2 minutes before the start of the case and is repeated verbally to the candidate when he or she enters the room. This is why it is important to practise short cases of every conceivable type so that the physical examination is performed automatically in the correct way. While proceeding, the candidate should be consciously synthesising the results, not trying to remember what to do next. In the short-case examination of a patient with bronchiectasis, the stem may be: `This man has a cough. Candidates who ask the patient to cough would pass, but those who do not ask him to cough would more probably not pass. In reality, there is plenty of time, but you certainly do not want to run out of time before getting to the problem the examiners have raised. The examiners will expect a higher standard of examination when the diagnosis is fairly obvious. Starting off It is a good idea, when introduced to the patient, to step over and shake his or her hand firmly. It is always worthwhile taking a moment to stand back and look at the whole patient. This may prevent you from missing an obvious spot diagnosis, such as myxoedema, a thymectomy scar in a patient with muscle weakness (myasthenia), muscle fasciculations in motor neurone disease, a psoriatic rash in a patient with arthropathy or a Cushingoid ht tp Wash your hands before and after examining. Some candidates keep a small bottle of hand gel in their bags so that they can be seen to wash their hands and are not delayed by having to search the room. The examiners do not usually want you to wash them in the examination room because of the delay involved, but look around for somewhere to wash as you go out. We suggest that candidates aim to examine each short case proficiently within 8 minutes. The examiners decide what signs they feel are most important and, in practice, finding the majority of the agreed signs will result in a pass. Performance Remember that you are, in fact, demonstrating the signs (particularly in the case of a neurological short case) to the examiners. Be seen to be smooth and confident, as if you have done the examination a thousand times before. This is not necessary unless the introduction mentioned hypertension (or you find hypertension when taking the blood pressure). In the past the measurement was often provided when the candidate asked, `Do you want me to take the blood pressure This is not as simple as it sounds and many candidates can have considerable difficulty with this. In practice examinations, some candidates have looked shocked when told to go ahead and do what they had just offered to do; others struggled with an unfamiliar sphygmomanometer, while others looked as though they did not really know how to measure blood pressure. For example: `I was asked to examine Mr Jones, a 60-year-old man who has had problems with dyspnoea. When describing the signs it is probably easiest to present them in the order they were looked for. There may not be time to complete a complicated examination, particularly in a neurology case. A good candidate will tailor the examination so that nothing really important is left out by the end of the time available. We suggest that candidates keep on examining until told to stop, and then list all the other things they would like to have done and why. Sometimes the examination seems to be going well and then the candidate comes out with a completely wrong diagnosis. In this case the examiner will likely attempt to continue the discussion along the lines the candidate has begun. For example, the candidate appears to have examined a patient with small muscle wasting of the hands satisfactorily, but then, against all the evidence, decides the problem is rheumatoid arthritis. The examiners may ask what was found that led to the diagnosis and were there any alternative possibilities, but if no alternatives can be extracted from the candidate they will allow a discussion of rheumatoid arthritis. For example, for an obvious acromegalic patient the stem might be: `This man has noticed some changes in his hands. The examination and discussion will then have nothing to do with what the examiners had expected and prepared for. Some candidates persist in continuing the way they began, despite strong hints or even direction from the examiners. For example, a patient with fasciculation plus upper and lower motor neurone signs in the legs and no sensory loss almost certainly has motor neurone disease, but a nonmetastatic manifestation of carcinoma must be considered. Never reel off any old list; the differential diagnosis must be tailored to the particular patient. After presentation of the signs, a few minutes or more are set aside for discussion.