Purchase 1 mg finasteride with visa
This may be one of the glucagon-like peptides released from the distal small intestine hair loss home remedies cheap finasteride 5mg mastercard. Trophic agents such as gastrin stimulate polyamine synthesis in the proliferative cells. Thus increased luminal polyamines from the diet, coupled with synthesis stimulated by trophic hormones, may explain some of the regulation of mucosal growth triggered by changes in the diet. The effects of hypophysectomy on growth can be prevented by administration of growth hormone. Gastrin is an important and necessary regulator of the growth of the oxyntic gland mucosa. It also stimulates growth of the intestinal and colonic mucosa and the exocrine pancreas. Disruption of gastrin gene expression in mice inhibits parietal cell maturation and decreases their number. Conversely, overexpression of the gene results in increased proliferation of the gastric epithelium, increased number of parietal cells, and increased acid secretion. Although the formation of both types of ulcers requires acid and pepsin, their causes are basically different. Quite simply, an ulcer forms when damage from acid and pepsin overcomes the ability of the mucosa to protect itself and replace damaged cells. In the case of gastric ulcer, the defect is more often in the ability of the mucosa to withstand injury. In the case of duodenal ulcer, good evidence indicates that the mucosa is exposed to increased amounts of acid and pepsin. This analysis is an oversimplification, because both factors are no doubt important in all cases of ulcer. Representative acid secretory rates for normal individuals and for patients with gastrointestinal disorders are shown in Table 8. Maximal acid secretory output sometimes is measured but in itself is of little value in diagnosing ulcer disease. Normal subjects secrete approximately 25 mEq hydrogen (H+)/hour, in response to maximal injection of histamine, betazole, or gastrin. The mean output of patients with duodenal ulcer disease is approximately 40 mEq H+/hour, but the degree of overlap among individuals is so great as to render the determination useless in diagnosis. The highest rates of acid secretion are seen in cases of gastrinoma (Zollinger-Ellison syndrome), but again individual overlap makes it impossible to differentiate between this condition and duodenal ulcer on the basis of secretory data alone. Lower than normal secretory rates are found in cases of gastric ulcer, and still lower secretory rates are found in patients with gastric carcinoma. Many patients in the latter two groups, however, fall well within the normal range. Plasma proteins and pepsin leak into the gastric juice; if damage is severe, bleeding will occur. Common agents that produce mucosal damage of this type are aspirin, ethanol, and bile salts. The mucosal lesions produced by topical damage to the gastric barrier may be forerunners of gastric ulcer. Factors that have been speculated to play a role in maintaining mucosal resistance are blood flow, mucus, bicarbonate secretion, cellular renewal, and chemical factors such as gastrin, prostaglandins, and epidermal growth factor. The last three agents have all been shown to decrease the severity and promote the healing of gastric ulcers. Factors that have been elucidated as important in duodenal ulcer formation pertain to acid and pepsin secretion. Patients with duodenal ulcer have on the average 2 billion parietal cells and can secrete approximately 40 mEq H+/hour. Comparable measurements for normal individuals are approximately 50% of this number. In addition, the secretion of pepsin is doubled in the duodenal ulcer group, as can be detected by measuring plasma pepsinogen. Although fasting serum gastrin is normal in patients with duodenal ulcer, the gastrin response to a meal and sensitivity to gastrin are increased. Increased serum gastrin after a meal is caused in part by the fact that acid suppresses gastrin release less effectively in patients with duodenal ulcer than in controls. The increased parietal cell mass may therefore be caused by the trophic effect of gastrin. The major acquired factor in the origin of both gastric ulcer and duodenal ulcer is the bacterium Helicobacter pylori. This reaction allows the bacterium to withstand the acid environment of the stomach and to colonize the mucosa. The value is determined by multiplying the hourly volume of gastric juice aspirated times the hydrogen ion concentration of the juice. Because of the feedback mechanism whereby antral acidification inhibits gastrin release, the general statement can be made that serum gastrin levels are related inversely to acid secretory capacity. Patients with gastric ulcer and carcinoma usually have higher than normal serum gastrin levels. Serum gastrin levels in pernicious anemia actually may approach those seen in gastrinoma. Obviously, patients with gastrinoma are an exception to this rule because their hypergastrinemia is derived not from the antrum but from a tumor not subject to inhibition by gastric acid. Except for the special tests mentioned in Chapter 1, serum gastrin levels cannot be used to differentiate various secretory abnormalities. The decreased rate of acid secretion experienced with gastric ulcer is caused in part by the failure to recover acid that has been secreted and then has leaked back across the damaged gastric mucosa. When the gastric mucosal barrier is weakened or damaged, H+ leaks into the mucosa in exchange for sodium (Na+). As H+ accumulates in the mucosa, intracellular buffers are saturated, and the intracellular pH decreases, thus resulting in injury and cell death. They then release histamine, which exacerbates the condition by acting on H1 receptors in the mucosal capillaries. The bacteria produce numerous other factors, such as platelet-activating factor and cytokines, that also damage cells. All these findings, except the increased maximal acid output in response to gastrin, totally disappeared following eradication of the H. Gastric ulcers are associated with diffuse gastritis, whereas duodenal ulcers are associated predominantly with the infection of the antrum. Medical treatment of duodenal ulcer disease usually consists of administering antacids to neutralize secreted acid or a histamine H2-receptor blocker to inhibit secretion. It is extremely effective in treating duodenal ulcers, even those caused by gastrinoma. With the advent of the H2 blockers and proton pump inhibitors, ulcers are now rarely treated by surgical intervention. This is best done by giving antibiotics in combination with omeprazole, which increases the susceptibility of the bacteria to antibiotic treatment. How is the gastric mucosa able to secrete 1 to 2 L of fluid per day at a pH of less than 1 Experiments and Observations on the Gastric Juice and the Physiology of Digestion. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. The function of the aqueous component is the neutralization of the duodenal contents. As such, it prevents damage to the duodenal mucosa by acid and pepsin and brings the pH of the contents into the optimal range for activity of the pancreatic enzymes. The enzymatic or protein component is a low-volume secretion containing enzymes for the digestion of all normal constituents of a meal. Unlike the enzymes secreted by the stomach and salivary glands, the pancreatic enzymes are essential to normal digestion and absorption. Each acinus is formed from several pyramidal acinar cells oriented with their apices toward the lumen.
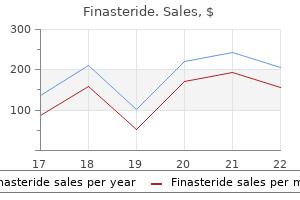
1mg finasteride sale
However hair loss gluten discount finasteride 5 mg mastercard, the numerous adverse reactions associated with oral therapy should be considered. Post-Marketing: Anaphylaxis within minutes to hours of infusion; angioedema involving the face, tongue, larynx, hands, and feet. Discontinue if hypercalcemia occurs, and institute appropriate measures to reduce the serum calcium level. Permanently discontinue if examination reveals papilledema or retinal vascular lesions. Manage toxicities with dose reduction, treatment delay, or discontinuation of copanlisib as outlined in the following chart. Withhold copanlisib until fasting glucose is 160 mg/dL or less or a random/nonfasting blood glucose is 200 mg/dL or less. Infections Hyperglycemia Predose or postdose blood glucose 500 mg/dL or more First occurrence: Withhold copanlisib until fasting blood glucose is 160 mg/dL or less or a random/ nonfasting blood glucose is 200 mg/dL or less. Subsequent occurrences: Withhold copanlisib as above, then reduce dose from 45 mg to 30 mg and maintain. Withhold copanlisib until toxicity is resolved, and reduce dose from 60 mg to 45 mg or from 45 mg to 30 mg. Withhold copanlisib and resume when platelet levels return to 75,000 cells/mm3 or greater. If recovery occurs within 21 days, reduce dose from 60 mg to 45 mg or from 45 mg to 30 mg. Repeat the gentle shaking and settling process if any undissolved substance is still seen. Use reconstituted and diluted solution immediately, or it may be refrigerated and used within 24 hours. Blood glucose levels typically peak 5 to 8 hours postinfusion and then decline to baseline levels in most patients. Maternal/Child: Based on findings in animals and on its mechanism of action, copanlisib can cause fetal harm. Elderly: No clinically relevant differences in efficacy observed between elderly patients and younger patients. Patients 65 years of age and older may have a greater incidence of serious reactions. Examples include carbamazepine (Tegretol), enzalutamide (Xtandi), mitotane (Lysodren), phenytoin (Dilantin), rifampin (Rifadin), and St. Hyperglycemia, hypertension, infections, neutropenia, noninfectious pneumonitis, and severe cutaneous reactions are serious adverse reactions. Laboratory abnormalities include decreased hemoglobin, lymphocytes, neutrophils, platelets, and white blood cells; hyperglycemia; hypertriglyceridemia; hyperuricemia; hypophosphatemia; and increased serum lipase. Oral pain, oropharyngeal erosion and ulcer, pneumonia (bacterial, fungal, pneumococcal, pneumocystis jiroveci, and viral), rash, stomatitis, and vomiting have also been reported. Administer appropriate treatment if an infusion reaction and/or a hypersensitivity reaction occur. Manufacturer states, "Should not be added to blood or plasma; may be inactivated by enzymes. A synthetic peptide that is an analog of the naturally occurring human corticotropin releasing hormone. Administer corticorelin and draw venous blood samples 15, 30, and 60 minutes after administration. Patient Education: Report dizziness, feeling faint, or shortness of breath immediately. A possible interaction between corticorelin and heparin may have been responsible for a major hypotensive reaction that occurred in study patients after corticorelin administration. Hypersensitivity reactions have been reported and may include flushing of the face, neck, and upper chest; dyspnea; wheezing; urticaria; and angioedema involving the tongue, lip, and facial swelling. Overdose: Doses over 1 mcg/kg may cause dyspnea, hypotension, prolonged flushing, tachycardia, and a feeling of compression or tightness in the chest. Does not increase cortisol secretion in patients with primary adrenocortical insufficiency. Diagnostic aid for screening patients presumed to have adrenocortical insufficiency. For example, cortisol binding globulin levels can be low in cirrhosis or nephrotic syndrome. Monitor: Collect a baseline blood sample of 6 to 7 mL before administration of cosyntropin. Collect a second blood sample 30 minutes and/or 60 minutes after administration; see prescribing information for sampling and blood storage requirements. Stimulated plasma cortisol levels of less than 18 to 20 mcg/dL at 30 or 60 minutes after cosyntropin administration are suggestive of adrenocortical insufficiency. Patients receiving cortisone, hydrocortisone, or spironolactone should omit their pretest doses on the day of testing. Alternatively, concomitant measurement of cortisol binding globulin at the time of testing can be done; if cortisol binding globulin levels are elevated, plasma total cortisol levels are considered inaccurate. Bradycardia, hypertension, peripheral edema, rash, and tachycardia have been reported. Adult and pediatric patients: Administer as soon as possible after a rattlesnake bite in patients who develop any signs of envenomation. The amount of antivenin required to treat a snakebite is highly variable and depends on venom burden, potency of the venom, and the time to health care presentation. Monitor the patient for at least 60 minutes after completion of the infusion for any hypersensitivity reaction and to determine that local signs of envenomation are not progressing, systemic symptoms have resolved, and coagulation parameters have normalized or are trending toward normal; see Monitor. Repeat doses: Administer an additional 10 vials if needed to arrest the progressive symptoms. Observation and late dosing: Monitor patients in a health care setting for at least 18 hours following the initial control of signs and symptoms. Re-emerging symptoms, including coagulopathies, may be suppressed with an additional 4 vials as needed. Antivenin dose following a snakebite is expected to be the same in pediatric patients and adults; no dose adjustment for age is required. Fluid volumes may need to be adjusted for very small children or infants; consult a regional poison control center. Infuse at 25 to 50 mL/hr for the first 10 minutes, carefully monitoring for any hypersensitivity reaction, including anaphylaxis. If no reactions occur, the infusion rate may be increased to deliver the full 250 mL/hr rate until completion. If there is any hypersensitivity reaction at any time, stop the infusion, treat accordingly, and reassess risk to benefit before continuing the infusion; see Antidote. Patients with known allergies to horse protein are at particular risk for an anaphylactic reaction. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient. Repeat as indicated to gauge response to therapy and anticipate additional dosing. Patient Education: Immediately report any S/S of delayed hypersensitivity reactions or serum sickness. The most common adverse reactions include arthralgia, erythema, headache, myalgia, nausea, pain in extremity, peripheral edema, pruritus, rash, and vomiting. Anxiety, blisters, chills, dehydration, dyspnea, fever, insomnia, and thrombocytopenia have also occurred. Keep the physician informed of all side effects and the extent or progression of envenomation.
Discount finasteride 1 mg with amex
Patients receiving liver transplants may be more susceptible to encephalopathy than patients receiving kidney transplants hair loss cure news 2013 purchase finasteride with american express. Reversal of encephalopathy has occurred after discontinuation or dose reduction of cyclospo- tion of a physician experienced in immunosuppressive therapy and management of organ transplant patients. Do not administer cyclosporine with any other immunosuppressive agent except adrenocortical steroids. Monitor continuously for the first 30 minutes of the infusion and frequently thereafter. Initiation or modification of antihypertensive therapy may be indicated; do not use potassium-sparing diuretics. Should not be used unless benefit to the mother justifies potential risk to the fetus. Reported outcomes of pregnancies in women who received cyclosporine are difficult to evaluate. It is not possible to separate the effects of cyclosporine from the effects of other medications, underlying maternal disorders, or other aspects of the transplantation process. Negative outcomes included prematurity, low birth weight, fetal loss, and various malformations. Accidental parenteral overdose in premature neonates has caused serious symptoms of intoxication. Other sources list acyclovir (Zovirax), foscarnet (Foscavir), selected quinolones. Cyclosporine may decrease the clearance and increase the toxic effects of colchicine. Compounds that decrease cyclosporine absorption, such as orlistat (Alli), should be avoided. Other sources list sulfamethoxazole/trimethoprim; monitor levels and adjust cyclosporine dose as indicated to avoid transplant rejection. May decrease the volume distribution of digoxin and cause toxicity rather quickly. With concurrent use, monitor digoxin levels, reduce digoxin dose, or discontinue as indicated. Statins may be temporarily withheld or discontinued in patients with S/S of myopathy or potential for renal injury, including renal failure, secondary to rhabdomyolysis. To minimize the effect on blood levels, administer sirolimus 4 hours after cyclosporine dose. Other side effects include acne, convulsions, cramps, diarrhea, encephalopathy, glomerular capillary thrombosis, headache, hepatotoxicity, hyperkalemia, hyperuricemia, hypomagnesemia, infection, leukopenia, lymphoma, microangiopathic hemolytic anemia, nausea and vomiting, paresthesia, skin rash, and thrombocytopenia. Treat hypersensitivity as indicated; may require oxygen, epinephrine (Adrenalin), antihistamines. Acute myelocytic leukemia or erythroleukemia in adult and pediatric patients: Manufacturer lists a dose of 100 mg/M2/24 hr as a continuous infusion or 200 mg/M2/day continuous infusion (as 100 mg/M2 over 12 hours every 12 hours) for 7 days. Through various chemical processes this deprivation acts more quickly on rapidly growing cells and causes their death. Used in combination with other approved anticancer drugs for remission induction in acute nonlymphocytic leukemia in adults and pediatric patients. Hypersensitivity to cytarabine, pre-existing drug-induced bone marrow suppression. Use with caution in patients with preexisting drug-induced bone marrow suppression. Less serious toxicity includes nausea, vomit- n Administered by or under the direction of a physician specialist in a facility with adequate diagnostic and treatment facilities to monitor the patient and respond to any medical emergency. Must be able to monitor drug tolerance and protect and maintain a patient compromised by drug toxicity. The main toxic effect of cytarabine is bone marrow suppression with leukopenia, thrombocytopenia, and anemia. Counts may continue to fall after the drug is stopped and may reach lowest values after drug-free intervals of 12 to 14 days. Patients whose drug is withheld until "normal" peripheral blood values are attained may escape from control. Can cause fetal harm when administered to a pregnant woman, especially during the first trimester. Drug must be restarted as soon as signs of bone marrow recovery occur, or its effectiveness will be lost. Use corticosteroids for cytarabine syndrome (fever, myalgia, bone pain, occasional chest pain, maculopapular rash, conjunctivitis, malaise). There is no specific antidote; supportive therapy as indicated will help to sustain the patient in toxicity. Additional infusions of 100 mg/kg are given at 2, 4, 6, and 8 weeks posttransplant, then reduced to 50 mg/kg at 12 and 16 weeks posttransplant. May be increased to 30 mg/kg/hr in 30 minutes if no discomfort or adverse effects. May be increased in another 30 minutes to 60 mg/kg/hr if no discomfort or adverse effects. Do not exceed the 60 mg/kg/hr rate or allow the volume infused to exceed 75 mL/hr regardless of mg/kg/hr dose. Subsequent doses may be increased at 15-minute intervals using the same mg/kg/hr rates and adhering to the volume maximum of 75 mL/hr. History of a prior severe reaction associated with any human immunoglobulin preparations. Individuals with selective immunoglobulin A deficiency may develop antibodies to IgA and are at risk for anaphylaxis. Effective results have been obtained with a variety of immunosuppressive regimens. Must be monitored before infusion, at every rate change, at the midpoint, at the conclusion, and several times after completion. Maternal/Child: Category C: safety for use during pregnancy or breast-feeding not established. Incidence related to rate of administration; back pain, chills, fever, flushing, hypotension, muscle cramps, nausea, vomiting, wheezing. May be treated symptomatically and infusion resumed at a slower rate if symptoms subside. An alternate regimen is 375 mg/M2 on Days 1 and 15 every 4 weeks or 100 mg/M2/day for 5 days. Unlabeled uses: Treatment of malignant pheochromocytoma with cyclophosphamide and vincristine. Patient Education: Protect skin surfaces; may cause photosensitive skin reactions. Maternal/Child: Category C: safety for use in pregnancy or breast-feeding and in men and women capable of conception not established. Monitor: Determine absolute patency of vein; a stinging or burning sensation indicates Follow guidelines for handling cytotoxic agents. Do not administer any live virus vaccines to patients receiving antineoplastic drugs. Effects may be decreased with carbamazepine (Tegretol), phenobarbital, and rifampin (Rifadin). Alopecia, anaphylaxis, anorexia, facial flushing, facial paresthesias, fever, hepatotoxicity, malaise, myalgia, nausea, skin necrosis, vomiting. Bone marrow suppression may require temporary or permanent withholding of treatment. Dose will depend on tolerance of the patient, the size and location of the tumor, and the use of other forms of therapy. Calculation of the dose for obese or edematous patients should be based on body surface area. The dose intensity per 2-week cycle for adult and pediatric patients should not exceed 15 mcg/kg/day (0. May be administered in various combinations and schedules with other chemotherapeutic agents. Metastatic nonseminomatous testicular cancer: 1,000 mcg/M2 (1 mg/M2) on Day 1 as part of a combination regimen with cyclophosphamide, bleomycin, vinblastine, and cisplatin. Gestational trophoblastic neoplasia: 12 mcg/kg/day for 5 days as a single agent or 500 mcg on Days 1 and 2 as part of a combination regimen with etoposide, methotrexate, folinic acid, vincristine, cyclophosphamide, and cisplatin.
1mg finasteride with mastercard
Monitor coagulation parameters frequently hair loss cure diet finasteride 5mg sale, and adjust anticoagulant dose accordingly. Aplastic anemia, erythema multiforme, hemolytic anemia, hemorrhage, hepatic dysfunction (including cholestasis), pancytopenia, renal dysfunction, Stevens-Johnson syndrome, toxic epidermal necrolysis, and toxic nephropathy have been reported with cephalosporin-class antibiotics. Fatal cases of ceftriaxone-calcium precipitates in lungs and kidneys of neonates have been reported; see Contraindications, Precautions, and Maternal/Child. Vitamin K may be useful in bleeding episodes, or drug may need to be discontinued. Uncomplicated infections (gonococcal, pneumonia, skin and soft tissue, urinary tract): 750 mg Severe or complicated infections and bone and joint infections: 1. Pediatric patients 3 months of age or older: 50 to 100 mg/kg/day in equally divided doses every 6 to 8 hours (12. Bacterial meningitis: 200 to 240 mg/kg/day in equally divided doses every 6 to 8 hours (50 to 60 mg/kg every 6 hours or 66. The American Academy of Pediatrics suggests the following doses: Neonates: 7 days of age or younger regardless of weight: 50 mg/kg every 12 hours. Neonates from 8 to 28 days of age weighing 2 kg or less: 50 mg/kg every 8 to 12 hours. Neonates from 8 to 28 days of age weighing more than 2 kg: 50 mg/kg every 8 hours. Reduced doses or extended intervals may be indicated in the elderly; consider age-related impaired organ function, nutritional status, and concomitant disease or drug therapy. Adults: Reduce total daily dose if renal function impaired according to the following chart. Cefuroxime Dose Guidelines in Impaired Renal Function in Adults Creatinine Clearance (mL/min). Manufacturer recommends temporarily discontinuing other solutions infusing at the same site during intermittent infusion and lists sodium bicarbonate incompatible as a diluent. Injection or intermittent infusion may be given through Y-tube or three-way stopcock of infusion set. Treatment of patients with infections caused by susceptible strains of designated organisms in the following diseases: serious lower respiratory tract, urinary tract, bone and joint, skin and skin structure infections, septicemia, and meningitis. Patients at risk include those with renal or hepatic impairment, patients with poor nutritional status, patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy; see Monitor. Abdominal pain, agranulocytosis, aplastic anemia, colitis, hemolytic anemia, hemorrhage, hepatic dysfunction (including cholestasis), pancytopenia, prolonged prothrombin time, renal dysfunction, toxic nephropathy, and vaginitis, including vaginal candidiasis, have been reported with cephalosporin-class antibiotics. Resume in patients with complete or partial resolution (Grade 0 or 1) after corticosteroid taper. Discard the vial if the solution is cloudy, discolored, or contains extraneous particulate matter other than trace amounts of translucent-to-white particles. After preparation, store the prepared infusion at room temperature for no more than 8 hours from the time of preparation to the end of the infusion, or refrigerate for no more than 24 hours from the time of preparation to the end of the infusion. If refrigerated, allow the diluted solution to come to room temperature before administration. Interrupt, slow the rate of infusion, or permanently discontinue based on severity of any infusion-related reaction; see Dose Adjustments and Antidote. Steady-state concentrations were reached after approximately 4 months of treatment when cemiplimab-rwlc was administered at a dose of 350 mg every 3 weeks. These reactions usually occur during treatment but can also manifest after discontinuation of therapy. Monitor: Obtain clinical chemistries, including liver and thyroid function tests, at baseline and periodically during treatment. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reaction is not controlled with corticosteroids. Administer hormone replacement therapy and corticosteroids as clinically indicated. Based on the severity of the adverse reaction, withhold or discontinue cemiplimab-rwlc, administer high-dose corticosteroids and, if appropriate, initiate hormone replacement therapy. Based on mechanism of action and data from animal studies, cemiplimab-rwlc can cause fetal harm when administered to a pregnant woman. Human IgG4 is known to cross the placental barrier, and cemiplimab-rwlc is an IgG4 immunoglobin. The most common Grade 3 to 4 adverse reactions were cellulitis, fatigue, hypertension, musculoskeletal pain, pneumonia, sepsis, skin infection, and urinary tract infection. Several immunemediated reactions have been reported, including colitis, dermatologic adverse reactions/ rash, endocrinopathies, hepatitis, nephritis and renal dysfunction, pneumonitis; see Precautions, Monitor, and Antidote. Other reported reactions include constipation, decreased appetite, nausea, and pruritus. Follow guidelines for withholding or discontinuing therapy as outlined in Dose Adjustments. Interrupt or slow the rate of administration in patients with mild or moderate infusion reactions. Discontinue in patients with a severe or life-threatening infusion reaction and treat as indicated. Initiate corticosteroid therapy as outlined in Monitor for immunemediated reactions. Preassessment required to determine appropriate patient selection for patients with colorectal cancer; baseline studies indicated; see Monitor. Premedication: To prevent or attenuate severe infusion reactions, premedicate with an H1 antagonist. Premedication for subsequent infusions should be based on clinical judgment and the presence/severity of previous infusion reactions. Initial dose: Entire contents of 3 vials is the recommended initial dose based on clinical experience. Repeat doses: If control of symptoms is not accomplished by the initial dose, infuse one vial at a time at intervals of 30 to 60 minutes. Absolute venom dose following scorpion sting is expected to be the same in pediatric patients and adults; no dose adjustment for age is required. For initial and repeat doses, the solution should be clear; do not use if turbidity is present. Binds and neutralizes venom toxins, facilitating redistribution away from target tissues and elimination from the body. Patients with a known allergy to horse protein are particularly at risk for anaphylaxis. Patients who have had a previous equine antivenom/ antitoxin may be sensitized to equine proteins and be at risk for severe hypersensitivity reactions. Patient Education: Promptly report any S/S of delayed hypersensitivity reactions or serum sickness. Less common side effects included cough, diarrhea, fatigue, headache, lethargy, myalgia, and rhinorrhea. Other rarely reported side effects included aspiration, ataxia, hypoxia, pneumonia, respiratory distress, serum sickness, and swelling of the eyes. Discontinue the drug and treat hypersensitivity reactions and/or anaphylaxis immediately with oxygen, epinephrine (Adrenalin), antihistamines. Squamous cell carcinoma of the head and neck as monotherapy: First infusion: 400 mg/M2 as an initial loading dose. Recommended Cetuximab Dosage Modifications for Adverse Reactions Adverse Reaction Severitya Grade 1 or 2 Grade 3 or 4 1st occurrence; Grade 3 or 4 2nd occurrence; Grade 3 or 4 3rd occurrence; Grade 3 or 4 4th occurrence; Grade 3 or 4 Dosage Modification Reduce infusion rate by 50%. Delay infusion 1 to 2 weeks; if condition improves, continue at the dose that was being administered at the time of occurrence. May pool volume required to provide calculated dose into an empty evacuated container. Solution is clear and may contain small amounts of easily visible white particulates.
Purchase finasteride from india
Elderly: All Products: Numbers in clinical studies insufficient to determine whether the elderly respond differently than do younger subjects hair loss in men alopecia order 5 mg finasteride mastercard. Breath odor, dizziness, dys- geusia, fatigue, hypotension, infusion site pain, obstructive uropathy, and palpitations have been reported. No inhibitors were detected and no events of anaphylaxis were reported during clinical studies. Apathy, asthenia, depression, dysgeusia, influenza, injection site discomfort, lethargy, and pruritic rash have been reported. Temporarily discontinue or decrease rate of administration for minor side effects. If any major symptoms appear, discontinue drug, notify physician, and consider alternative hemostatic measures. Remove flip caps on vial and diluent, and wipe stoppers with provided alcohol swab; allow to dry. Peel away the lid on the Mix2Vial transfer set, but leave it in the clear package. Hold the diluent vial tightly on a flat surface and pick up the Mix2Vial transfer set by its clear package. Push the plastic spike at the blue end of the Mix2Vial transfer set through the center of the diluent vial stopper. Keep the syringe plunger pressed, and invert the system upside down to draw the concentrate into the syringe by pulling the plunger back slowly. If the same patient is to receive more than one vial, contents of multiple vials may be pooled. Several manufacturing steps are used to inactivate or remove both enveloped and nonenveloped viruses. Cross-linked fibrin is the end result of the coagulation cascade and provides tensile strength to a primary hemostatic platelet plug. Limitation of use: There are no controlled trials demonstrating a direct benefit on treatment of bleeding episodes. Hypersensitivity reactions have occurred; emergency equipment, medications, and supplies must be available. Assess benefit versus risk in pregnant women because of their hypercoagulable state and potential for increased risk of thromboembolic events. No apparent differences in the safety profile in pediatric patients compared with adults. Pediatric patients under 16 years of age had a shorter half-life and faster clearance compared with adults. The most commonly reported side effects included arthralgia, chills, elevated thrombinantithrombin levels, fever, headache, hypersensitivity reactions (including allergy, erythema, pruritus, and rash), and increased hepatic enzymes. Treatment duration and dose must be individualized based on clinical response, pH determination, and/or endoscopy. Reduce dose by one-half or increase the dosing interval to 36 to 48 hours in patients with moderate or severe renal dysfunction (CrCl less than 50 mL/min). Manufacturer recommends use of diluted solu- infusion solution and given piggyback. Short-term treatment of active duodenal ulcers, benign gastric ulcers, and pathologic hypersecretory conditions in hospitalized patients or in patients unable to take oral medication. Patient Education: Stop smoking or at least avoid smoking after last dose of the day. Plasma clearance is reduced and half-life is increased in pediatric patients under 3 months of age compared to older pediatric patients with pharmacokinetic parameters similar to adults. Consider risk of renal dysfunction; reduced doses and monitoring of renal function may be indicated; see Dose Adjustments. May decrease cyclosporine serum levels when famotidine is given concurrently with ketoconazole and cyclosporine. Hypersensitivity reactions (bronchospasm, fever, pruritus, rash, eosinophilia) can occur. For partial parenteral nutrition, energy supplied by oral or enteral nutrition must be taken into account. Essential fatty acid deficiency (Intralipid and Nutrilipid): 8% to 10% of the caloric input should be supplied as a fat emulsion to provide adequate amounts of essential fatty acids; see Dose Adjustments. The American Society of Enteral and Parenteral Nutrition recommends the following doses for pediatric patients. Caloric source (as a component of parenteral nutrition): Infants (preterm and term to less than 1 year of age): 1 to 2 Gm/kg/day as an initial dose. Adolescents: 1 Gm/kg/day as an initial dose (not to exceed 500 mL of Intralipid 20% on the first day). Begin with Lower initial starting doses and smaller incremental advances are suggested in patients with elevated triglyceride levels. If essential fatty acid deficiency occurs together with stress, dose may need to be increased. Must be given as prepared by manufacturer; check labels for aluminum content; see Precautions. When combined with dextrose, check mixture closely for the presence of precipitates. Manufacturer recommends not mixing with any electrolyte or other nutrient solution. Infuse separately; do not disturb emulsion; no additives or medications are to be placed in bottle or tubing with the exception of heparin 1 to 2 units/mL (may be added before administration [activates lipoprotein lipase]). In actual practice, carbohydrates, amino acids, and fat emulsion are mixed in specific percentages and in a specific order to meet individual total parenteral nutritional needs but should be prepared in the pharmacy. The prime destabilizers of emulsions are excessive acidity (such as pH below 5) and inappropriate electrolyte content. Divalent cations (calcium and magnesium) have been shown to cause emulsion instability. Rates of both solutions (fat emulsion and amino acid products) should be controlled by infusion pumps. In general, administration rate should be adjusted taking into account the dose being administered, the daily volume intake, and the duration of the infusion. If no untoward effects after 30 minutes, the rate may be increased gradually to the required rate (Nutrilipid: 0. Fatty acids are important for membrane structure and function as precursors for bioactive molecules. The fatty acids, phospholipids, and glycerol found in lipid emulsions are metabolized by cells to carbon dioxide and water. To provide additional calories and essential fatty acids for patients requiring parenteral nutrition who will be receiving parenteral nutrition over extended periods (usually over 5 days) or when oral or enteral nutrition is not possible, is insufficient, or is contraindicated. Limitation of use: Clinolipid is not indicated for use in pediatric patients because there are insufficient data to demonstrate that it provides sufficient amounts of essential fatty acids in this population. Severe hyperlipidemia (serum triglycerides above 1,000 mg/dL) or severe disorders of lipid metabolism, such as pathologic hyperlipemia, lipoid nephrosis, and acute pancreatitis with hyperlipemia. Use caution in pulmonary disease, liver disease, anemia, or blood coagulation disorders or when there is any danger of fat embolism. Patients requiring parenteral nutrition may be at higher risk for infection due to malnutrition, underlying disease state, and/or catheter access. Administration of parenteral nutrition to severely undernourished patients may result in refeeding syndrome. Correct severe water and electrolyte disorders, severe fluid overload states, and severe metabolic disorders before administration. For severely undernourished patients initiating parenteral nutrition therapy, monitor closely for S/S of refeeding syndrome. May be characterized by the intracellular shift of potassium, phosphorus, and magnesium; thiamine deficiency; and fluid retention. Maternal/Child: Category C: use in pregnancy only when clearly needed; safety not established. Severe malnutrition in a pregnant woman is associated with preterm delivery, low birth weight, intrauterine growth restriction, congenital malformations, and perinatal mortality. Use extreme caution in neonates; death from intravascular fat accumulation in the lungs has occurred.
Cheap 5 mg finasteride with visa
Neonatal: Bradycardia hair loss in men kissing cheap 5mg finasteride visa, coarse tremors, hypertonicity alternating with hypotonicity, hypotension, intracranial hemorrhage, opisthotonic posturing, and severe acidosis. The dose expressed as caffeine base is one-half the dose when expressed as caffeine citrate. The recommended loading dose and maintenance dose are listed in the following chart. Guidelines for Loading and Maintenance Doses of Caffeine Citrate Dose of Cafcit (volume) Loading dose Maintenance dose 1 mL/kg 0. Withdraw calculated dose and dilute with sufficient D5W to administer at the recommended rate of administration. Stable for 24 hours at room temperature when mixed with any of the solutions listed by the manufacturer in Compatibility. Metabolism and elimination in the preterm neonate are much slower than in adults due to immature hepatic and/or renal function. Mean half-life and fraction excreted unchanged in the urine is inversely related to gestational/postconceptual age. In neonates, the half-life is approximately 3 to 4 days and the fraction excreted unchanged in the urine is approximately 86% (within 6 days). By 9 months of age, the metabolism of caffeine approximates that seen in adults (half-life is 5 hours and amount excreted unchanged is 1%). Interconversion between caffeine and theophylline has been reported in preterm neonates. After theophylline administration, caffeine levels are approximately 25% of theophylline levels. After caffeine administration, 3% to 5% of caffeine administered converts to theophylline. Necrotizing enterocolitis, resulting in death in some cases, has been reported in neonates receiving caffeine citrate. Monitor: Obtain baseline serum caffeine levels in infants previously treated with theophylline because preterm infants metabolize theophylline to caffeine; see Actions. Levels should also be obtained in infants born to mothers who ingested caffeine before delivery, as caffeine readily crosses the placenta. A therapeutic plasma concentration range has not been determined, but one source suggests 5 to 25 mcg/mL. Monitoring is especially important in infants with impaired renal or hepatic function; see Dose Adjustments. Screening stools for occult blood may be helpful in identifying early-onset necrotizing enterocolitis. Patient Education: Caregivers should be instructed to consult physician if infant continues to have apnea events and to not increase the dose of caffeine citrate without consulting a physician. Lower caffeine doses may be required with coadministration of medications that inhibit the P450 system, decreasing the elimination of caffeine. Higher caffeine doses may be needed with coadministration of medications that induce the P450 system, increasing the elimination of caffeine. Seizures may be treated with intravenous administration of diazepam (Valium) or a barbiturate such as pentobarbital (Nembutal). Calaspargase-Pegol-mknl Dose Modifications for Adverse Reactions Adverse Reaction Infusion reaction or hypersensitivity reaction Severitya Grade 1 Grade 2 Action Reduce the infusion rate by 50%. Resume calaspargase pegol-mknl with the next scheduled dose if bleeding is controlled. Discontinue calaspargase pegol-mknl permanently if clinical pancreatitis is confirmed. Upon resolution of symptoms, consider resuming calasparagase pegol-mknl while continuing antithrombotic therapy. Grade 3 to 4 Hemorrhage Grade 3 to 4 Pancreatitis Grade 3 to 4 Thromboembolism Uncomplicated deep vein thrombosis Severe or lifethreatening thrombosis Hepatoxicity Total bilirubin. Calaspargase pegol-mknl contains an asparagine-specific, enzyme-derived Escherichia coli as a conjugate of l-asparaginase and monomethoxypolyethylene glycol with a succinimidyl carbonate linker. The pharmacologic effect of calaspargase pegol-mknl is thought to be based on selective killing of leukemic cells due to depletion of plasma l-asparagine. Leukemic cells with low expression of asparagine synthetase have a reduced ability to synthesize l-asparagine and therefore depend on an exogenous source of l-asparagine for survival. Patients with a history of any of the following: serious hypersensitivity reactions, including anaphylaxis, to pegylated l-asparaginase therapy; serious thrombosis during previous l-asparaginase therapy; serious pancreatitis during previous l-asparaginase therapy; serious hemorrhagic events during previous l-asparaginase therapy. Monitor: Monitor patients at least weekly, with bilirubin, transaminases, glucose, and clinical examinations until recovery from cycle of therapy. Assess serum amylase and/or lipase levels to confirm early signs of pancreatic inflammation. Patient Education: Promptly report all side effects, especially unusual bleeding or bruising, excessive thirst, frequent urination, hypersensitivity reactions. Maternal/Child: Based on animal studies, calaspargase pegol-mknl can cause fetal harm when administered to a pregnant woman. The most common adverse reactions were abnormal clotting studies, increased bilirubin, elevated transaminase, and pancreatitis. Other side effects include arrhythmia, cardiac failure, diarrhea, dyspnea, embolic and thrombotic events, fungal infection, hemorrhages, hypersensitivity, pneumonia, and sepsis. Discontinue in patients who experience a serious hypersensitivity reaction or a serious thrombotic event. If serious liver toxicity occurs, discontinue treatment and provide supportive care. Consider appropriate replacement therapy in patients with severe or symptomatic coagulopathy. Calcium supplementation or proper dietary measures must be initiated and maintained. Hypocalcemia and/or secondary hyperparathyroidism: Recommended initial dose, depending on the severity of hypocalcemia and/or secondary hyperparathyroidism, is 1 mcg (0. Information supplied by the manufacturer suggests that the relative dosing of paricalcitol to calcitriol is 4:1. Monitor serum calcium, phosphorus, and calcium 3 phosphorus product (Ca 3 P) frequently during any dose adjustment period; see Monitor. Calcitriol may be drawn up into a syringe up to 8 hours before administration but must be protected from direct sunlight. Must be metabolically activated in liver and kidney before it is fully active on its target tissues. Because calcitriol is the most potent form of vitamin D available, oral vitamin D supplements should be discontinued during treatment. Therefore gradual titration downward to a new maintenance dose is recommended; see Dose Adjustments. Chronic hypercalcemia can lead to generalized vascular calcification, nephrocalcinosis, and other soft-tissue calcification. Radiographic evaluation of suspect anatomic regions may be useful in early detection of this condition; see Side Effects and Antidote. A decision should be made whether to discontinue nursing or to discontinue the drug. Consider age-related organ impairment, concomitant disease, and/or drug therapy; see Dose Adjustments. High intake of calcium and phosphate concomitant with calcitriol therapy may lead to similar abnormalities. Signs and symptoms of vitamin D intoxication associated with hypercalcemia include Early: bone pain, constipation, dry mouth, headache, metallic taste, muscle pain, nausea, somnolence, vomiting, and weakness. Rare cases of hypersensitivity reactions, including anaphylaxis, have been reported.
Purchase finasteride australia
Discontinue eravacycline at the first sign of a hypersensitivity reaction or if the development of any tetracycline-class adverse drug reaction is suspected hair loss in men zara discount finasteride 5mg line. If toxicities do not resolve or improve to Grade 2 or less by Day 15, omit the dose. If toxicities resolve or improve to Grade 2 or less by Day 15, administer at a reduced dose as outlined in the following chart and initiate the next cycle no sooner than 2 weeks later. Recommended Dose Reductions for Eribulin Mesylate Event Description Recommended Eribulin Dose 1. Do not administer in the same intravenous line concurrent with other medicinal products. Inhibits the growth phase of microtubules without affecting the shortening phase and sequesters tubulin into nonproductive aggregates. Exerts its effects via a tubulin-based antimitotic mechanism leading to G2/M cell-cycle block, disruption of mitotic spindles and, ultimately, apoptotic cell death after prolonged mitotic blockage. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting. Deaths from complications of febrile neutropenia and neutropenic sepsis have been reported. In clinical trials, the mean time to nadir was 13 days, and the mean time to recovery from severe neutropenia was 8 days. Peripheral neuropathy was the most common toxicity leading to discontinuation of therapy. Increase frequency of hematologic monitoring in patients who develop severe cytopenias (Grade 3 or 4); see Dose Adjustments. Correct hypokalemia or hypomagnesemia before initiating therapy, and monitor electrolytes periodically during therapy. Use prophylactic antiemetics to reduce nausea and vomiting and increase patient comfort. Women should use effective contraception during treatment with eribulin and for 2 weeks following the final dose. Males with female partners of reproductive potential should use effective contraception during treatment and for 3. Elderly: No overall differences in safety were observed between older and younger patients. The most common side effects resulting in discontinuation of eribulin were fatigue, peripheral neuropathy, and thrombocytopenia. Grade 3 or 4 laboratory abnormalities included hypocalcemia, hypokalemia, and neutropenia. Less frequently reported side effects included anxiety, depression, dizziness, dry mouth, dysgeusia, dyspepsia, hyperglycemia, hypophosphatemia, hypotension, increased lacrimation, insomnia, muscle spasm or weakness, oropharyngeal pain, peripheral edema, rash, stomatitis, and upper respiratory tract infection. Post-Marketing: Dehydration, hepatotoxicity, hypersensitivity, hypomagnesemia, interstitial lung disease, lymphopenia, neutropenic sepsis, pancreatitis, pneumonia, pruritus, sepsis, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Give a supplementary dose of 150 mg to patients who received the daily dose within 6 hours of a dialysis session. No data are available for pediatric patients with renal insufficiency or pediatric patients on hemodialysis. The pharmacokinetics of ertapenem in patients with hepatic impairment have not been established. A unique, synthetic 1-beta-methyl-carbapenem structurally related to beta-lactam antibiotics. Stable against hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases, and extended-spectrum beta-lactamases. Known hypersensitivity to any component of ertapenem or other drugs in the same class. More likely in patients with a history of sensitivity to multiple allergens; obtain a careful history and watch for early symptoms of hypersensitivity reactions. Consider in patients who present with diarrhea during or after treatment with ertapenem. If focal tremors, myoclonus, or seizures occur, neurologic evaluation and dose reduction or discontinuation of ertapenem may be indicated. Maternal/Child: Available data insufficient to inform any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes. If administration of ertapenem is necessary, supplemental anticonvulsant therapy should be considered. Other side effects that were reported in greater than 2% of patients included abdominal pain, altered mental status, constipation, dizziness, dyspnea, edema/swelling, fever, hypotension, insomnia, pruritus, rash, and vomiting. Antibacterial: 15 to 20 mg/kg of body weight/24 hr in equally divided doses every 6 hours (3. When practical, continue treatment with oral erythromycin 3 times daily, 30 minutes before meals, for 4 weeks. Another source recommends 20 to 50 mg/kg/24 hr in equally divided doses every 6 hours (5 to 12. If a dextrose solution is used, add sodium bicarbonate (Neut) 1 mL for each 100 mL of solution. Intermittent infusion: 1 Gm or fraction thereof in at least 100 mL over 20 to 60 minutes. Diabetic gastroparesis (unlabeled): 1 to 3 mg/kg/hr, usually approximately over 15 minutes. Effective against a number of grampositive and some gram-negative organisms as well as Mycoplasma pneumoniae. Inhibits protein synthesis by binding to ribosomal subunits of susceptible organisms. Contraindicated with astemizole and terfenadine (both have been removed from the market). Elderly: When doses of 4 Gm/day or higher are used, the risk of developing erythromycininduced hearing loss is increased in elderly patients, particularly those with impaired renal or hepatic function. For severe symptoms, discontinue the drug, treat hypersensitivity reactions, or resuscitate as necessary and notify physician. Additional loading doses and/or titration of the maintenance infusion (stepwise dosing) may be necessary based on desired ventricular response. Effective maintenance dose for continuous and stepwise dosing is 50 to 200 mcg/kg/min, although doses as low as 25 mcg/kg/min have been adequate. Doses greater than 200 mcg/kg/min provide little additional lowering of heart rate, and the rate of adverse reactions increases. Intraoperative and postoperative tachycardia and/or hypertension: Immediate control: 1 mg/kg over 30 seconds. Maintenance infusion doses greater than 200 mcg/kg/min are not recommended for the treatment of tachycardia. They provide little additional lowering of heart rate, and the rate of adverse reactions increases. After administration of the second dose of the alternative drug, monitor patient response carefully. If control is satisfactory and is maintained for 1 hour, discontinue the esmolol infusion. Antiarrhythmic (unlabeled): Pediatric patients 1 to 12 years of age: A loading dose of 100 to 500 mcg/kg (0. Doses as high as 1,000 mcg/kg/min have been administered to pediatric patients 1 to 12 years of age. Antihypertensive (postoperative [unlabeled]): Pediatric patients 1 to 12 years of age: A loading dose of 500 mcg/kg (0. No dose adjustment required if the maintenance infusion does not exceed 150 mcg/kg/min for more than 4 hours; no data available for higher doses or longer duration. Premixed solutions have a delivery port and a medication port (for withdrawing the initial bolus only). If a bolus has been removed from the premixed bag, the bag should be used within 24 hours. Half-life is approximately 9 minutes, and the effects last about 20 to 30 minutes. Management of supraventricular tachycardia (atrial fibrillation or atrial flutter) in situations requiring short-term control of ventricular rate with a short-acting agent (perioperative, postoperative, or other emergent circumstances). Risk is increased in patients with renal impairment and is potentially life threatening in hemodialysis patients.